Simultaneous point-of-care testing of blood lipid profile and glucose: Performance evaluation of the GCare Lipid Analyzer
- PMID: 34697832
- PMCID: PMC8649338
- DOI: 10.1002/jcla.24055
Simultaneous point-of-care testing of blood lipid profile and glucose: Performance evaluation of the GCare Lipid Analyzer
Abstract
Background: Point-of-care (POC) testing provides quick results and includes tests for blood glucose and lipid profiles. We evaluated the newly developed POC device, the GCare Lipid Analyzer, which is used to measure glucose, total cholesterol (TC), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) levels.
Methods: Venous and capillary blood samples were collected from patients who visited Korea University Guro Hospital. The results obtained using the GCare Lipid Analyzer were compared with those obtained using the TBA 2000FR chemistry analyzer and YSI 2300 STAT Plus analyzer. The glucose system evaluation process was based on the International Organization for Standardization 15197:2013 guidelines.
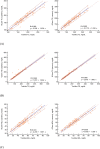
Results: The correlation coefficients (R) for TC, TG, and HDL-C were 0.965, 0.969, and 0.943 in capillary blood and 0.969, 0.990, and 0.956 in venous blood, respectively. The total errors for TC, TG, and HDL-C of the lipid profile using venous blood were all acceptable at 6.6%, 9.3%, and 11.6%, respectively. For glucose concentrations <100 mg/dl, 96.1% of the measured glucose levels were within ±15 mg/dl in venous samples and 100% were within ±15 mg/dl in capillary samples. For glucose concentrations ≥100 mg/dl, 100% and 99.5% of the measured glucose levels were within 15% for venous and capillary blood, respectively.
Conclusion: The performance of the GCare Lipid Analyzer is acceptable for both blood glucose and lipid profile testing, indicating that it is reliable for use in patients with diabetic dyslipidemia.
Keywords: POC; diabetes mellitus; dyslipidemia; glucose; lipid; point-of-care testing.
© 2021 The Authors. Journal of Clinical Laboratory Analysis published by Wiley Periodicals LLC.
Conflict of interest statement
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



Similar articles
-
Performance evaluation of three i-SENS glucometers using arterial blood samples compared with the YSI 2300 Glucose Analyzer.J Clin Lab Anal. 2020 Aug;34(8):e23356. doi: 10.1002/jcla.23356. Epub 2020 May 19. J Clin Lab Anal. 2020. PMID: 32430994 Free PMC article.
-
Comparison of two point-of-care lipid analyzers for use in global cardiovascular risk assessments.Ann Pharmacother. 2008 May;42(5):633-9. doi: 10.1345/aph.1K688. Epub 2008 Apr 15. Ann Pharmacother. 2008. PMID: 18413684
-
[Study on the reliability of CardioChek PA for measuring lipid profile].Beijing Da Xue Xue Bao Yi Xue Ban. 2016 Jun 18;48(3):523-8. Beijing Da Xue Xue Bao Yi Xue Ban. 2016. PMID: 27318918 Chinese.
-
Comparing the Accuracy of 2 Point-of-Care Lipid Testing Devices.J Pharm Pract. 2017 Oct;30(5):490-497. doi: 10.1177/0897190016651546. Epub 2016 Jun 16. J Pharm Pract. 2017. PMID: 27313238
-
Maternal lipid profile and risk of pre-eclampsia in African pregnant women: A systematic review and meta-analysis.PLoS One. 2020 Dec 23;15(12):e0243538. doi: 10.1371/journal.pone.0243538. eCollection 2020. PLoS One. 2020. PMID: 33362205 Free PMC article.
Cited by
-
Diagnostic accuracy of triglyceride to glucose index and triglyceride/high-density lipoprotein index for insulin resistance among children and adolescents: A systematic review.PLoS One. 2025 Jun 25;20(6):e0326179. doi: 10.1371/journal.pone.0326179. eCollection 2025. PLoS One. 2025. PMID: 40560922 Free PMC article.
References
-
- Grundy SM. Diabetes and coronary risk equivalency ‐ what does it mean? Diabetes Care. 2006;29(2):457‐460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

