Using inpatient electronic medical records to study influenza for pandemic preparedness
- PMID: 34697904
- PMCID: PMC8818824
- DOI: 10.1111/irv.12921
Using inpatient electronic medical records to study influenza for pandemic preparedness
Abstract
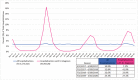
Background: We assessed the ability to identify key data relevant to influenza and other respiratory virus surveillance in a large-scale US-based hospital electronic medical record (EMR) dataset using seasonal influenza as a use case. We describe characteristics and outcomes of hospitalized influenza cases across three seasons.
Methods: We identified patients with an influenza diagnosis between March 2017 and March 2020 in 140 US hospitals as part of the US FDA's Sentinel System. We calculated descriptive statistics on the presence of high-risk conditions, influenza antiviral administrations, and severity endpoints.
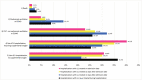
Results: Among 5.1 million hospitalizations, we identified 29,520 hospitalizations with an influenza diagnosis; 64% were treated with an influenza antiviral within 2 days of admission, and 25% were treated >2 days after admission. Patients treated >2 days after admission had more comorbidities than patients treated within 2 days of admission. Patients never treated during hospitalization had more documentation of cardiovascular and other diseases than treated patients. We observed more severe endpoints in patients never treated (death = 3%, mechanical ventilation [MV] = 9%, intensive care unit [ICU] = 26%) or patients treated >2 days after admission (death = 2%, MV = 14%, ICU = 32%) than in patients treated earlier (treated on admission: death = 1%, MV = 5%, ICU = 23%, treated within 2 days of admission: death = 1%, MV = 7%, ICU = 27%).
Conclusions: We identified important trends in influenza severity related to treatment timing in a large inpatient dataset, laying the groundwork for the use of this and other inpatient EMR data for influenza and other respiratory virus surveillance.
Keywords: electronic medical records; influenza hospitalizations; public health surveillance.
© 2021 The Authors. Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Pottegård A, Kurz X, Moore N, Christiansen CF, Klungel O. Considerations for pharmacoepidemiological analyses in the SARS‐CoV‐2 pandemic. Pharmacoepidemiol Drug Saf. 2020;29(8):825‐831. - PubMed
-
- Curtis LH, Brown J, Platt R. Four health data networks illustrate the potential for a shared national multipurpose big‐data network. Health Aff Proj Hope. 2014;33(7):1178‐1186. - PubMed
-
- Eggleston EM, Weitzman ER. Innovative uses of electronic health records and social media for public health surveillance. Curr Diab Rep. 2014;14(3):468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

