Fusion of apoptosis-related protein Cytochrome c with anti-HER-2 single-chain antibody targets the suppression of HER-2+ breast cancer
- PMID: 34697906
- PMCID: PMC8581304
- DOI: 10.1111/jcmm.17001
Fusion of apoptosis-related protein Cytochrome c with anti-HER-2 single-chain antibody targets the suppression of HER-2+ breast cancer
Abstract
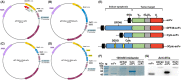
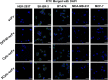
Cancer treatment has gradually developed from toxic chemotherapy to targeted therapy with fewer side effects. Approximately 30% of breast cancer patients overexpress human epidermal growth factor receptor 2 (HER-2). Previous studies have successfully produced single-chain antibodies (scFv) targeting HER-2+ breast cancer; however, scFv have poor stability, easy aggregation and a shorter half-life, which have no significant effect on targeting therapy. Moreover, scFv has been considered as a drug delivery platform that can kill target cells by effector molecules. However, the functional killing domains of immunotoxins are mainly derived from plant or bacterial toxins, which have a large molecular weight, low tissue permeability and severe side effects. To address these concerns, we designed several apoptotic immune molecules to replace exogenous toxins using endogenous apoptosis-related protein DNA fragmentation factor 40 (DFF40) and tandem-repeat Cytochrome c base on caspase-3 responsive peptide (DEVD). Our results suggest that DFF40 or Cytc fusion scFv specifically targets HER-2 overexpressing breast cancer cells (SK-BR-3 and BT-474) rather than HER-2 negative cells (MDA-MB-231 and MCF-7). Following cellular internalization, apoptosis-related proteins inhibited tumour activity by initiating endogenous apoptosis pathways, which significantly reduced immunogenicity and toxic side effects. Therefore, we suggest that immunoapoptotic molecules may become potential drugs for targeted immunotherapy of breast cancer.
Keywords: HER-2; breast cancer; immunoapoptotic molecules; scFv; targeted therapy.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare no financial competing interests. All authors declare no nonfinancial competing interests.
Figures




Similar articles
-
DNA fragmentation factor 40-based therapeutic approaches for cancer: a review article.Med Oncol. 2024 Oct 14;41(11):264. doi: 10.1007/s12032-024-02511-5. Med Oncol. 2024. PMID: 39397131 Review.
-
Antitumor activity and toxicity of anti-HER2 immunoRNase scFv 4D5-dibarnase in mice bearing human breast cancer xenografts.Invest New Drugs. 2011 Feb;29(1):22-32. doi: 10.1007/s10637-009-9329-2. Epub 2009 Sep 30. Invest New Drugs. 2011. PMID: 19789841
-
Granzyme B-based cytolytic fusion protein targeting EpCAM specifically kills triple negative breast cancer cells in vitro and inhibits tumor growth in a subcutaneous mouse tumor model.Cancer Lett. 2016 Mar 28;372(2):201-9. doi: 10.1016/j.canlet.2016.01.027. Epub 2016 Jan 21. Cancer Lett. 2016. PMID: 26806809
-
Development of a human immuno-oncology therapeutic agent targeting HER2: targeted delivery of granzyme B.J Exp Clin Cancer Res. 2019 Jul 30;38(1):332. doi: 10.1186/s13046-019-1333-6. J Exp Clin Cancer Res. 2019. PMID: 31362764 Free PMC article.
-
Designing the Sniper: Improving Targeted Human Cytolytic Fusion Proteins for Anti-Cancer Therapy via Molecular Simulation.Biomedicines. 2017 Feb 17;5(1):9. doi: 10.3390/biomedicines5010009. Biomedicines. 2017. PMID: 28536352 Free PMC article. Review.
Cited by
-
The Impact of Long Noncoding RNAs in Tissue Regeneration and Senescence.Cells. 2024 Jan 9;13(2):119. doi: 10.3390/cells13020119. Cells. 2024. PMID: 38247811 Free PMC article. Review.
-
Unlocking the potential of engineered microbes in immunotoxin-based cancer therapy.Front Microbiol. 2025 Jun 5;16:1603671. doi: 10.3389/fmicb.2025.1603671. eCollection 2025. Front Microbiol. 2025. PMID: 40539104 Free PMC article. Review.
-
DNA fragmentation factor 40-based therapeutic approaches for cancer: a review article.Med Oncol. 2024 Oct 14;41(11):264. doi: 10.1007/s12032-024-02511-5. Med Oncol. 2024. PMID: 39397131 Review.
-
A novel shark VNAR antibody-based immunotoxin targeting TROP-2 for cancer therapy.Acta Pharm Sin B. 2024 Nov;14(11):4806-4818. doi: 10.1016/j.apsb.2024.08.023. Epub 2024 Aug 27. Acta Pharm Sin B. 2024. PMID: 39664437 Free PMC article.
-
Cytochrome c in cancer therapy and prognosis.Biosci Rep. 2022 Dec 22;42(12):BSR20222171. doi: 10.1042/BSR20222171. Biosci Rep. 2022. PMID: 36479932 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7‐34. - PubMed
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7‐30. - PubMed
-
- Falo C, Moreno A, Varela M, Lloveras B, Figueras A, Escobedo A. HER‐2/neu status and response to CMF: retrospective study in a series of operable breast cancer treated with primary CMF chemotherapy. J Cancer Res Clin Oncol. 2007;133:423‐429. - PubMed
-
- Harbeck N. Advances in targeting HER2‐positive breast cancer. Curr Opin Obstet Gynecol. 2018;30:55‐59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

