Increased Hepatitis C virus screening, diagnosis and linkage to care rates among people who use drugs through a patient-centered program from Italy
- PMID: 34697911
- PMCID: PMC8672087
- DOI: 10.1002/ueg2.12156
Increased Hepatitis C virus screening, diagnosis and linkage to care rates among people who use drugs through a patient-centered program from Italy
Abstract
Background: Rates of Hepatitis C virus (HCV) testing and diagnosis are variable among people who use drugs (PWUD). In Puglia in 2018, of 871 subjects screened, 38% had HCV antibodies (HCVAb). Despite sustained virologic response at week 12 Sustained virologic response (SVR12) rates >95%, addiction centers in Italy are not allowed to prescribe direct-acting antivirals (DAA).
Aim: To increase testing and linkage to care a dedicated program including "ad hoc" transportation and fast-track access to care was offered to PWUD from Puglia.
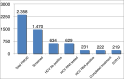
Methods: Over 12 months, 1,470 individuals seen at 15 Services for Dependence (SERDs) underwent screening. For HCVAb positive, a fast-track evaluation was offered at our Hepatology Unit. Patients were subsequently taken to their pharmacists to receive the prescribed DAA regimen. Treatment and adherence were supervised by SERDs physicians, SVR12 assessed at our unit. The scalability of the process was based on both, number of patients screened in our region in 2018, and number of PWUD diagnosed and treated at our center during 2018-2019.
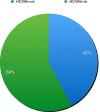
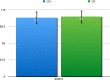
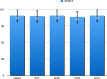
Results: Of 1,470 individuals screened, 634 (43.1%) tested HCVAb positive. Overall, 231 were RNA positive, 54% of whom on opioid agonist therapy (OAT) and 32% with cirrhosis. Median interval between RNA assessment and treatment start was 22 days (0-300). Patients received 12-week sofosbuvir/velpatasvir regimen without Ribavirin; in 220 patients who completed treatment, SVR12 was 98.6%. Among GT3, SVR12 was 98%. No re-infection was observed. Improvements in screening, and linkage to care were registered.
Conclusions: A PWUD-tailored service led to HCV care cascade improvement and high SVR12 rates. Despite history of drug addiction, social instability and logistic barriers, micro-elimination programs providing dedicated care are key drivers of success.
Trial registration: ClinicalTrials.gov NCT03923595.
Keywords: HCV; PWUD; antiviral treatment; cirrhosis; hepatitis C; linkage to care; micro-elimination; people who use drugs; screening; sofosbuvir/velpatasvir.
© 2021 The Authors. United European Gastroenterology Journal published by Wiley Periodicals LLC. on behalf of United European Gastroenterology.
Conflict of interest statement
Maria Franca Rina, Antonio Canosa, Valeria Piazzolla, Maria Maddalena Squillante, Ernesto Agostinacchio, Giovanna Cocomazzi, Egidio Visaggi, Nazario Augello, Camilla Iannuzziello, Mattia Falcone, Angelo De Giorgi, Fausto Campanozzi: None. Alessandra Mangia disclose fees for consulting, speakers bureaus and research support from BMS, Gilead, MSD, Intercept.
Figures
Comment in
-
If the mountain won't come to Mohammed, then Mohammed must go to the mountain: Decentralisation of hepatitis C care for people who use drugs.United European Gastroenterol J. 2021 Dec;9(10):1101-1102. doi: 10.1002/ueg2.12167. Epub 2021 Oct 22. United European Gastroenterol J. 2021. PMID: 34687156 Free PMC article. No abstract available.
References
-
- World Health Organization. Global health sector strategy on viral hepatitis 2016–21. https://www.who.int/hepatitis/strategy2016‐2021/ghss‐hep/en/. Accessed January 2021.
-
- AASLD . Call to action for liver associations to advance progress towards viral hepatitis elimination: a focus on simplified approaches to HCV testing and cure. AASLD; 2019.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

