Diverse vaccine platforms safeguarding against SARS-CoV-2 and its variants
- PMID: 34697980
- PMCID: PMC8787839
- DOI: 10.1080/14760584.2022.1997601
Diverse vaccine platforms safeguarding against SARS-CoV-2 and its variants
Abstract
Introduction: Appearances of SARS-CoV-2 variants have created havoc and additional challenges for the ongoing vaccination drive against pandemic COVID-19. Interestingly, several vaccine platforms are showing great potential to produce successful vaccines against SARS-CoV-2 and its variants. Billions of COVID-19 vaccine doses have been administered worldwide. Mix-and-Match COVID-19 vaccines involving the mixing of the same platform vaccines and also two different vaccine platforms may provide greater protection against SARS-CoV-2 and its variants. COVID-19 vaccines have become one of the most important tools to mitigate the ongoing pandemic COVID-19.
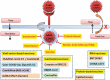
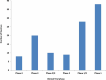
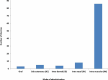
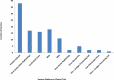
Areas covered: We describe SARS-Cov-2 variants, their impact on the population, COVID-19 vaccines, diverse vaccine platforms, doses of vaccines, the efficacy of vaccines against SARS-CoV-2 and its variants, mitigation of the COVID-19 transmission- alternatives to vaccines.
Expert opinion: Diverse vaccine platforms may safeguard against ongoing, deadly SARS-CoV-2 and its infectious variants. The efficacies of COVID-19 vaccines are significantly high after the administration of the second dose. Further, it protects individuals including vulnerable patients with co-morbidities from SARS-CoV-2 and its variants. The hospitalizations and deaths of the individuals may be prevented by COVID-19 vaccines.
Keywords: COVID-19; diverse; platforms; sars-cov-2; vaccines; variants.
Figures


References
-
- Thakur SS. Proteomics and Its Application in Pandemic Diseases. J Proteome Res. 2020;19(11):4215–4218. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
