Empowering Melatonin Therapeutics with Drosophila Models
- PMID: 34698120
- PMCID: PMC8544433
- DOI: 10.3390/diseases9040067
Empowering Melatonin Therapeutics with Drosophila Models
Abstract
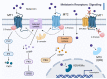
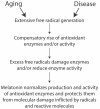
Melatonin functions as a central regulator of cell and organismal function as well as a neurohormone involved in several processes, e.g., the regulation of the circadian rhythm, sleep, aging, oxidative response, and more. As such, it holds immense pharmacological potential. Receptor-mediated melatonin function mainly occurs through MT1 and MT2, conserved amongst mammals. Other melatonin-binding proteins exist. Non-receptor-mediated activities involve regulating the mitochondrial function and antioxidant cascade, which are frequently affected by normal aging as well as disease. Several pathologies display diseased or dysfunctional mitochondria, suggesting melatonin may be used therapeutically. Drosophila models have extensively been employed to study disease pathogenesis and discover new drugs. Here, we review the multiple functions of melatonin through the lens of functional conservation and model organism research to empower potential melatonin therapeutics to treat neurodegenerative and renal diseases.
Keywords: Drosophila; PKD; longevity; melatonin; neurodegeneration; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Chowdhury I., Sengupta A., Maitra S.K. Melatonin: Fifty years of scientific journey from the discovery in bovine pineal gland to delineation of functions in human. Ind. J. Biochem. Biophys. 2008;45:289–304. - PubMed
-
- Lerner A.B., Case J.D., Takahashi Y., Lee T.H., Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958;80:2587. doi: 10.1021/ja01543a060. - DOI
-
- Dubbels R., Reiter R.J., Klenke E., Goebel A., Schnakenberg E., Ehlers C., Schiwara H.W., Schloot W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995;18:28–31. doi: 10.1111/j.1600-079X.1995.tb00136.x. - DOI - PubMed
-
- Hattori A., Migitaka H., Iigo M., Itoh M., Yamamoto K., Ohtani-Kaneko R., Hara M., Suzuki T., Reiter R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995;35:627–634. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

