Formulation and In-Vitro Characterization of pH-Responsive Semi-Interpenetrating Polymer Network Hydrogels for Controlled Release of Ketorolac Tromethamine
- PMID: 34698162
- PMCID: PMC8544598
- DOI: 10.3390/gels7040167
Formulation and In-Vitro Characterization of pH-Responsive Semi-Interpenetrating Polymer Network Hydrogels for Controlled Release of Ketorolac Tromethamine
Abstract
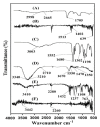
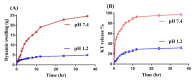
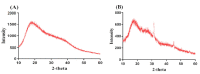
Ketorolac tromethamine is a non-steroidal anti-inflammatory drug used in the management of severe pain. The half-life of Ketorolac tromethamine is within the range of 2.5-4 h. Hence, repeated doses of Ketorolac tromethamine are needed in a day to maintain the therapeutic level. However, taking several doses of Ketorolac tromethamine in a day generates certain complications, such as acute renal failure and gastrointestinal ulceration. Therefore, a polymeric-controlled drug delivery system is needed that could prolong the release of Ketorolac tromethamine. Therefore, in the current study, pH-responsive carbopol 934/sodium polystyrene sulfonate-co-poly(acrylic acid) (CP/SpScPAA) hydrogels were developed by the free radical polymerization technique for the controlled release of Ketorolac tromethamine. Monomer acrylic acid was crosslinked with the polymers carbopol 934 and sodium polystyrene sulfonate by the cross-linker N',N'-methylene bisacrylamide. Various studies were conducted to evaluate and assess the various parameters of the fabricated hydrogels. The compatibility of the constituents used in the preparation of hydrogels was confirmed by FTIR analysis, whereas the thermal stability of the unreacted polymers and developed hydrogels was analyzed by TGA and DSC, respectively. A smooth and porous surface was indicated by SEM. The crystallinity of carbopol 934, sodium polystyrene sulfonate, and the prepared hydrogels was evaluated by PXRD, which revealed a reduction in the crystallinity of reactants for the developed hydrogels. The pH sensitivity of the polymeric hydrogel networks was confirmed by dynamic swelling and in vitro release studies with two different pH media i.e., pH 1.2 and 7.4, respectively. Maximum swelling was exhibited at pH 7.4 compared to pH 1.2 and, likewise, a greater percent drug release was perceived at pH 7.4. Conclusively, we can demonstrate that the developed pH-sensitive hydrogel network could be employed as a suitable carrier for the controlled delivery of Ketorolac tromethamine.
Keywords: carbopol 934; hydrogel; ketorolac tromethamine; sodium polystyrene sulfonate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Jeganath S., Asha D., Kumar S.S., Nair K.S., Kumaran K.S. Oral Controlled Drug Delivery System—A Review. Res. J. Pharm. Technol. 2018;11:797–804. doi: 10.5958/0974-360X.2018.00151.8. - DOI
-
- Khan S., Minhas M.U., Ahmad M., Sohail M. Self-assembled supramolecular thermoreversible beta-cyclodextrin/ethylene glycol injectable hydrogels with difunctional Pluronic((R))127 as controlled delivery depot of curcumin. Development, characterization and in vitro evaluation. J. Biomater. Sci. Polym. Ed. 2018;29:1–34. doi: 10.1080/09205063.2017.1396707. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

