Instantaneous Degelling Thermoresponsive Hydrogel
- PMID: 34698198
- PMCID: PMC8544516
- DOI: 10.3390/gels7040169
Instantaneous Degelling Thermoresponsive Hydrogel
Abstract
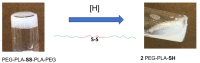
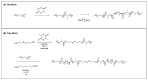
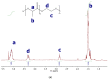
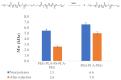
Responsive polymeric hydrogels have found wide application in the clinic as injectable, biocompatible, and biodegradable materials capable of controlled release of therapeutics. In this article, we introduce a thermoresponsive polymer hydrogel bearing covalent disulfide bonds. The cold aqueous polymer solution forms a hydrogel upon heating to physiological temperatures and undergoes slow degradation by hydrolytic cleavage of ester bonds. The disulfide functionality allows for immediate reductive cleavage of the redox-sensitive bond embedded within the polymer structure, affording the option of instantaneous hydrogel collapse. Poly(ethylene glycol)-b-poly(lactic acid)-S-S-poly(lactic acid)-b-poly(ethylene glycol) (PEG-PLA-SS-PLA-PEG) copolymer was synthesized by grafting PEG to PLA-SS-PLA via urethane linkages. The aqueous solution of the resultant copolymer was a free-flowing solution at ambient temperatures and formed a hydrogel above 32 °C. The immediate collapsibility of the hydrogel was displayed via reaction with NaBH4 as a relatively strong reducing agent, yet stability was displayed even in glutathione solution, in which the polymer degraded slowly by hydrolytic degradation. The polymeric hydrogel is capable of either long-term or immediate degradation and thus represents an attractive candidate as a biocompatible material for the controlled release of drugs.
Keywords: PEG-PLA; redox-sensitive; thermoresponsive hydrogel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



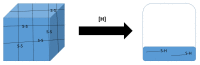
References
-
- Steinman N.Y., Haim-Zada M., Goldstein I.A., Goldberg A.H., Haber T., Berlin J.M., Domb A.J. Effect of PLGA block molecular weight on gelling temperature of PLGA-PEG-PLGA thermoresponsive copolymers. J. Polym. Sci. Part A Polym. Chem. 2019;57:35–39. doi: 10.1002/pola.29275. - DOI
LinkOut - more resources
Full Text Sources

