The role of vascular smooth muscle cells in the development of aortic aneurysms and dissections
- PMID: 34698377
- PMCID: PMC9285394
- DOI: 10.1111/eci.13697
The role of vascular smooth muscle cells in the development of aortic aneurysms and dissections
Abstract
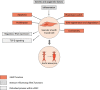
Background: Aortic aneurysms (AA) are pathological dilations of the aorta, associated with an overall mortality rate up to 90% in case of rupture. In addition to dilation, the aortic layers can separate by a tear within the layers, defined as aortic dissections (AD). Vascular smooth muscle cells (vSMC) are the predominant cell type within the aortic wall and dysregulation of vSMC functions contributes to AA and AD development and progression. However, since the exact underlying mechanism is poorly understood, finding potential therapeutic targets for AA and AD is challenging and surgery remains the only treatment option.
Methods: In this review, we summarize current knowledge about vSMC functions within the aortic wall and give an overview of how vSMC functions are altered in AA and AD pathogenesis, organized per anatomical location (abdominal or thoracic aorta).
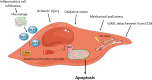
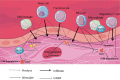
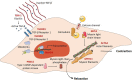
Results: Important functions of vSMC in healthy or diseased conditions are apoptosis, phenotypic switch, extracellular matrix regeneration and degradation, proliferation and contractility. Stressors within the aortic wall, including inflammatory cell infiltration and (epi)genetic changes, modulate vSMC functions and cause disturbance of processes within vSMC, such as changes in TGF-β signalling and regulatory RNA expression.
Conclusion: This review underscores a central role of vSMC dysfunction in abdominal and thoracic AA and AD development and progression. Further research focused on vSMC dysfunction in the aortic wall is necessary to find potential targets for noninvasive AA and AD treatment options.
Keywords: aortic aneurysm; aortic dissection; pathophysiology; vascular biology; vascular smooth muscle cell.
© 2021 The Authors. European Journal of Clinical Investigation published by John Wiley & Sons Ltd on behalf of Stichting European Society for Clinical Investigation Journal Foundation.
Conflict of interest statement
Not applicable.
Figures


Similar articles
-
[Aortic dissection and vascular smooth muscle cell apoptosis: in-depth exploration of their relationship and potential therapeutic strategies].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2025 Mar;37(3):225-231. doi: 10.3760/cma.j.cn121430-20241015-00842. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2025. PMID: 40201991 Review. Chinese.
-
Smad2-dependent protease nexin-1 overexpression differentiates chronic aneurysms from acute dissections of human ascending aorta.Arterioscler Thromb Vasc Biol. 2013 Sep;33(9):2222-32. doi: 10.1161/ATVBAHA.113.301327. Epub 2013 Jun 27. Arterioscler Thromb Vasc Biol. 2013. PMID: 23814118
-
Dexamethasone attenuated thoracic aortic aneurysm and dissection in vascular smooth muscle cell Tgfbr2-disrupted mice with CCL8 suppression.Exp Physiol. 2022 Jun;107(6):631-645. doi: 10.1113/EP090190. Epub 2022 May 26. Exp Physiol. 2022. PMID: 35344629
-
Vascular smooth muscle cell phenotypic changes in patients with Marfan syndrome.Arterioscler Thromb Vasc Biol. 2015 Apr;35(4):960-72. doi: 10.1161/ATVBAHA.114.304412. Epub 2015 Jan 15. Arterioscler Thromb Vasc Biol. 2015. PMID: 25593132
-
From genetics to response to injury: vascular smooth muscle cells in aneurysms and dissections of the ascending aorta.Cardiovasc Res. 2018 Mar 15;114(4):578-589. doi: 10.1093/cvr/cvy006. Cardiovasc Res. 2018. PMID: 29360940 Free PMC article. Review.
Cited by
-
The role of RUNX1/NF-κB in regulating PVAT inflammation in aortic dissection.Sci Rep. 2024 Apr 30;14(1):9960. doi: 10.1038/s41598-024-60737-9. Sci Rep. 2024. PMID: 38693222 Free PMC article.
-
Unveiling cellular and molecular aspects of ascending thoracic aortic aneurysms and dissections.Basic Res Cardiol. 2024 Jun;119(3):371-395. doi: 10.1007/s00395-024-01053-1. Epub 2024 May 3. Basic Res Cardiol. 2024. PMID: 38700707 Free PMC article. Review.
-
Influence of DNA Methylation on Vascular Smooth Muscle Cell Phenotypic Switching.Int J Mol Sci. 2024 Mar 8;25(6):3136. doi: 10.3390/ijms25063136. Int J Mol Sci. 2024. PMID: 38542110 Free PMC article. Review.
-
Agathis dammara Extract and its Monomer Araucarone Attenuate Abdominal Aortic Aneurysm in Mice.Cardiovasc Drugs Ther. 2025 Apr;39(2):239-257. doi: 10.1007/s10557-023-07518-0. Epub 2023 Nov 18. Cardiovasc Drugs Ther. 2025. PMID: 37979015
-
Independent and Interactive Roles of Immunity and Metabolism in Aortic Dissection.Int J Mol Sci. 2023 Nov 2;24(21):15908. doi: 10.3390/ijms242115908. Int J Mol Sci. 2023. PMID: 37958896 Free PMC article. Review.
References
-
- Lederle FA, Johnson GR, Wilson SE, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Ann Intern Med. 1997;126:441. - PubMed
-
- Blanchard JF, Armenian HK, Friesen PP. Risk factors for abdominal aortic aneurysm: results of a case‐control study. Am J Epidemiol. 1999;151(6):575‐583. - PubMed
-
- Milewicz DM, Guo DC, Tran‐Fadulu V, et al. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet. 2008;9:283‐302. - PubMed
-
- Assar AN, Zarins CK. Ruptured abdominal aortic aneurysm: a surgical emergency with many clinical presentations. Postgrad Med J. 2009;85(1003):268‐273. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

