Physiological roles of mammalian transmembrane adenylyl cyclase isoforms
- PMID: 34698552
- PMCID: PMC8759965
- DOI: 10.1152/physrev.00013.2021
Physiological roles of mammalian transmembrane adenylyl cyclase isoforms
Abstract
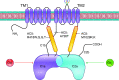
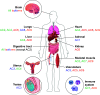
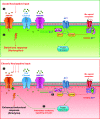
Adenylyl cyclases (ACs) catalyze the conversion of ATP to the ubiquitous second messenger cAMP. Mammals possess nine isoforms of transmembrane ACs, dubbed AC1-9, that serve as major effector enzymes of G protein-coupled receptors (GPCRs). The transmembrane ACs display varying expression patterns across tissues, giving the potential for them to have a wide array of physiological roles. Cells express multiple AC isoforms, implying that ACs have redundant functions. Furthermore, all transmembrane ACs are activated by Gαs, so it was long assumed that all ACs are activated by Gαs-coupled GPCRs. AC isoforms partition to different microdomains of the plasma membrane and form prearranged signaling complexes with specific GPCRs that contribute to cAMP signaling compartments. This compartmentation allows for a diversity of cellular and physiological responses by enabling unique signaling events to be triggered by different pools of cAMP. Isoform-specific pharmacological activators or inhibitors are lacking for most ACs, making knockdown and overexpression the primary tools for examining the physiological roles of a given isoform. Much progress has been made in understanding the physiological effects mediated through individual transmembrane ACs. GPCR-AC-cAMP signaling pathways play significant roles in regulating functions of every cell and tissue, so understanding each AC isoform's role holds potential for uncovering new approaches for treating a vast array of pathophysiological conditions.
Keywords: G protein-coupled receptors; adenylyl cyclase; cAMP.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

