Effect of Platelet-Rich Plasma Injections vs Placebo on Ankle Symptoms and Function in Patients With Ankle Osteoarthritis: A Randomized Clinical Trial
- PMID: 34698782
- PMCID: PMC8548954
- DOI: 10.1001/jama.2021.16602
Effect of Platelet-Rich Plasma Injections vs Placebo on Ankle Symptoms and Function in Patients With Ankle Osteoarthritis: A Randomized Clinical Trial
Abstract
Importance: Approximately 3.4% of adults have ankle (tibiotalar) osteoarthritis and, among younger patients, ankle osteoarthritis is more common than knee and hip osteoarthritis. Few effective nonsurgical interventions exist, but platelet-rich plasma (PRP) injections are widely used, with some evidence of efficacy in knee osteoarthritis.
Objective: To determine the effect of PRP injections on symptoms and function in patients with ankle osteoarthritis.
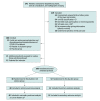
Design, setting, and participants: A multicenter, block-randomized, double-blinded, placebo-controlled clinical trial performed at 6 sites in the Netherlands that included 100 patients with pain greater than 40 on a visual analog scale (range, 0-100) and tibiotalar joint space narrowing. Enrollment began on August 24, 2018, and follow-up was completed on December 3, 2020.
Interventions: Patients were randomly assigned (1:1) to receive 2 ultrasonography-guided intra-articular injections of either PRP (n = 48) or placebo (saline; n = 52).
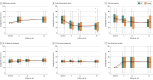
Main outcomes and measures: The primary outcome was the validated American Orthopaedic Foot and Ankle Society score (range, 0-100; higher scores indicate less pain and better function; minimal clinically important difference, 12 points) over 26 weeks.
Results: Among 100 randomized patients (mean age, 56 years; 45 [45%] women), no patients were lost to follow-up for the primary outcome. Compared with baseline values, the mean American Orthopaedic Foot and Ankle Society score improved by 10 points in the PRP group (from 63 to 73 points [95% CI, 6-14]; P < .001) and 11 points in the placebo group (from 64 to 75 points [95% CI, 7-15]; P < .001). The adjusted between-group difference over 26 weeks was -1 ([95% CI, -6 to 3]; P = .56). One serious adverse event was reported in the placebo group, which was unrelated to the intervention; there were 13 other adverse events in the PRP group and 8 in the placebo group.
Conclusions and relevance: Among patients with ankle osteoarthritis, intra-articular PRP injections, compared with placebo injections, did not significantly improve ankle symptoms and function over 26 weeks. The results of this study do not support the use of PRP injections for ankle osteoarthritis.
Trial registration: Netherlands Trial Register: NTR7261.
Conflict of interest statement
Figures


Comment in
-
Platelet-Rich Plasma Injections vs Placebo for Patients With Ankle Osteoarthritis.JAMA. 2022 Feb 22;327(8):777-779. doi: 10.1001/jama.2021.24751. JAMA. 2022. PMID: 35191931 No abstract available.
-
Platelet-Rich Plasma Injections vs Placebo for Patients With Ankle Osteoarthritis.JAMA. 2022 Feb 22;327(8):779. doi: 10.1001/jama.2021.24745. JAMA. 2022. PMID: 35191932 No abstract available.
-
Platelet-Rich Plasma Injections vs Placebo for Patients With Ankle Osteoarthritis.JAMA. 2022 Feb 22;327(8):779-780. doi: 10.1001/jama.2021.24742. JAMA. 2022. PMID: 35191933 No abstract available.
-
Platelet-Rich Plasma Injections vs Placebo for Patients With Ankle Osteoarthritis.JAMA. 2022 Feb 22;327(8):780. doi: 10.1001/jama.2021.24736. JAMA. 2022. PMID: 35191935 No abstract available.
References
-
- Murray C, Marshall M, Rathod T, Bowen CJ, Menz HB, Roddy E. Population prevalence and distribution of ankle pain and symptomatic radiographic ankle osteoarthritis in community dwelling older adults: a systematic review and cross-sectional study. PLoS One. 2018;13(4):e0193662. doi:10.1371/journal.pone.0193662 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

