Physician Perspectives on Including Pregnant Women in Covid-19 Clinical Trials: Time for a Paradigm Change
- PMID: 34699138
- PMCID: PMC8652879
- DOI: 10.1002/eahr.500107
Physician Perspectives on Including Pregnant Women in Covid-19 Clinical Trials: Time for a Paradigm Change
Abstract
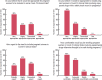
Excluding pregnant people from Covid-19 clinical trials may lead to unintended harmful consequences. For this study, an online questionnaire was sent to physicians belonging to Canadian professional medical associations in order to evaluate their perspectives on the participation of pregnant women in Covid-19 clinical trials. The majority of respondents expressed support for including pregnant women in Covid-19 trials (119/165; 72%), especially those investigating therapies with a prior safety record in pregnancy (139/164; 85%). The main perceived barriers to inclusion identified were unwillingness of pregnant patients to participate and of treating teams to offer participation, the burden of regulatory approval, and a general "culture of exclusion" of pregnant women from trials. We describe why some physicians may be reluctant to include pregnant individuals in trials, and we identify barriers to the appropriate participation of pregnant people in clinical research.
Keywords: Covid-19 clinical trials; human subjects research; inclusion of pregnant women in trials; maternal-fetal ethics; pregnant research participants; research with pregnant women.
© 2021 by The Hastings Center. All rights reserved.
Figures
References
-
- Committee on Ethics of the American College of Obstetricians and Gynecologists , “Ethical Considerations for Including Women as Research Participants. Committee Opinion No. 646,” Obstetrics & Gynecology 126 (2015): 1127–28; Shields, K. E., and A. D. Lyerly, “Exclusion of Pregnant Women from Industry‐Sponsored Clinical Trials,” Obstetrics & Gynecology 122 (2013): 1077–81. - PubMed
-
- Malhamé, I. , D'Souza R., and Cheng M. P., “The Moral Imperative to Include Pregnant Women in Clinical Trials of Interventions for COVID‐19,” Annals of Internal Medicine (2020): doi.org/10.7326/M20-3106. - PMC - PubMed
-
- Baylis, F. , and Halperin S., “Research Involving Pregnant Women: Trials and Tribulations,” Clinical Investigation 2, no. 2 (2012): 139–46.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical