Time-Resolved ATR-FTIR Spectroscopy and Macro ATR-FTIR Spectroscopic Imaging of Inorganic Treatments for Stone Conservation
- PMID: 34699174
- PMCID: PMC9295121
- DOI: 10.1021/acs.analchem.1c02392
Time-Resolved ATR-FTIR Spectroscopy and Macro ATR-FTIR Spectroscopic Imaging of Inorganic Treatments for Stone Conservation
Abstract
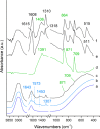
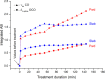
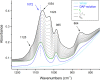
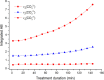
In this study, the novel application of ATR-FTIR spectroscopy and macro ATR-FTIR spectroscopic imaging overcame an analytical challenge in conservation science: the time-resolved, chemical, and spatial investigation of the reaction of inorganic treatments for stone conservation (ammonium oxalate, AmOx; ammonium phosphate, DAP) occurring in water-based solutions. The aim was to (1) assess the composition and localization of reaction products and their phase variation during the reaction in real time and directly in an aqueous environment and (2) investigate the reaction of AmOx and DAP with calcite and the transformations induced to the substrate with a time-resolved approach. The new analytical results showed that for both treatments, the formation of new crystalline phases initiated at the early stages of the reaction. Their composition changed during the treatment and led to more stable phases. The reactivity of the stone substrate to the treatments varied as a function of the stone material features, such as the specific surface area. A clear influence of post-treatment rinsing on the final composition of reaction phases was observed. Above all, our research demonstrates the actual feasibility, practicality, and high potential of an advanced ATR-FTIR spectroscopic approach to investigate the behavior of conservation treatments and provided new analytical tools to address the choices of conservation in pilot worksites. Lastly, this study opens novel analytical perspectives based on the new possible applications of ATR-FTIR spectroscopic imaging in the field of conservation science, materials science, and analytical chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Matteini M.; Rescic S.; Fratini F.; Botticelli G. Ammonium Phosphates as Consolidating Agents for Carbonatic Stone Materials Used in Architecture and Cultural Heritage: Preliminary Research. Int. J. Archit. Herit. Conserv. Anal. Restor. 2011, 5, 717–736. 10.1080/15583058.2010.495445. - DOI
-
- Possenti E.; Colombo C.; Conti C.; Gigli L.; Merlini M.; Plaisier J. R.; Realini M.; Sali D.; Gatta G. D. Diammonium Hydrogenphosphate for the Consolidation of Building Materials. Investigation of Newly-Formed Calcium Phosphates. Constr. Build. Mater. 2019, 195, 557–563. 10.1016/j.conbuildmat.2018.11.077. - DOI
-
- Possenti E.; Colombo C.; Conti C.; Marinoni N.; Merlini M.; Negrotti R.; Realini M.; Gatta G. D. Consolidation of Building Materials with a Phosphate-Based Treatment: Effects on the Microstructure and on the 3D Pore Network. Mater. Charact. 2019, 154, 315–324. 10.1016/j.matchar.2019.05.037. - DOI
-
- Possenti E. Inorganic Products Used in the Conservation of Cultural Heritage: Interaction with Carbonatic Substrates and Newly-Formed Crystalline Phases. Plinius 2019, 45, 60–67. 10.19276/plinius.2019.01009. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

