Phase II Randomized Trial of Transoral Surgery and Low-Dose Intensity Modulated Radiation Therapy in Resectable p16+ Locally Advanced Oropharynx Cancer: An ECOG-ACRIN Cancer Research Group Trial (E3311)
- PMID: 34699271
- PMCID: PMC8718241
- DOI: 10.1200/JCO.21.01752
Phase II Randomized Trial of Transoral Surgery and Low-Dose Intensity Modulated Radiation Therapy in Resectable p16+ Locally Advanced Oropharynx Cancer: An ECOG-ACRIN Cancer Research Group Trial (E3311)
Abstract
Purpose: Definitive or postoperative chemoradiation (CRT) is curative for human papillomavirus-associated (HPV+) oropharynx cancer (OPC) but induces significant toxicity. As a deintensification strategy, we studied primary transoral surgery (TOS) and reduced postoperative radiation therapy (RT) in intermediate-risk HPV+ OPC.
Methods: E3311 is a phase II randomized trial of reduced- or standard-dose postoperative RT for resected stage III-IVa (American Joint Committee on Cancer-seventh edition) HPV+ OPC, determined by pathologic parameters. Primary goals were feasibility of prospective multi-institutional study of TOS for HPV+ OPC, and oncologic efficacy (2-year progression-free survival) of TOS and adjuvant therapy in intermediate-risk patients after resection. TOS plus 50 Gy was considered promising if the lower limit of the exact 90% binomial confidence intervals exceeded 85%. Quality of life and swallowing were measured by functional assessment of cancer therapy-head and neck and MD Anderson Dysphagia Index.
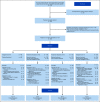
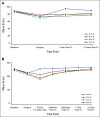
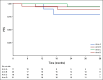
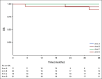
Results: Credentialed surgeons performed TOS for 495 patients. Eligible and treated patients were assigned as follows: arm A (low risk, n = 38) enrolled 11%, intermediate risk arms B (50 Gy, n = 100) or C (60 Gy, n = 108) randomly allocated 58%, and arm D (high risk, n = 113) enrolled 31%. With a median 35.2-month follow-up for 359 evaluable (eligible and treated) patients, 2-year progression-free survival Kaplan-Meier estimate is 96.9% (90% CI, 91.9 to 100) for arm A (observation), 94.9% (90% CI, 91.3 to 98.6]) for arm B (50 Gy), 96.0% (90% CI, 92.8 to 99.3) for arm C (60 Gy), and 90.7% (90% CI, 86.2 to 95.4) for arm D (66 Gy plus weekly cisplatin). Treatment arm distribution and oncologic outcome for ineligible or step 2 untreated patients (n = 136) mirrored the 359 evaluable patients. Exploratory comparison of functional assessment of cancer therapy-head and neck total scores between arms B and C is presented.
Conclusion: Primary TOS and reduced postoperative RT result in outstanding oncologic outcome and favorable functional outcomes in intermediate-risk HPV+ OPC.
Conflict of interest statement
Figures




Comment in
-
Transoral Surgery and Deintensified Adjuvant Therapy: Another Step in Determining Its Role in the Management of Human Papillomavirus Oropharyngeal Cancer.J Clin Oncol. 2022 Jan 10;40(2):114-117. doi: 10.1200/JCO.21.02364. Epub 2021 Nov 12. J Clin Oncol. 2022. PMID: 34767480 No abstract available.
-
[De-escalation of adjuvant radiotherapy after transoral surgery of HPV-associated oropharyngeal cancer: results of the E3311 trial].Strahlenther Onkol. 2022 Apr;198(4):400-403. doi: 10.1007/s00066-022-01907-4. Epub 2022 Feb 8. Strahlenther Onkol. 2022. PMID: 35137242 Free PMC article. German. No abstract available.
-
Transoral Surgery With Neck Dissection Is an Excellent Treatment for the Appropriately Selected Patient With Early-Stage Oropharyngeal Cancer.J Clin Oncol. 2022 Apr 1;40(10):1132-1133. doi: 10.1200/JCO.21.02630. Epub 2022 Feb 16. J Clin Oncol. 2022. PMID: 35171686 No abstract available.
-
Reply to A.S. Garden.J Clin Oncol. 2022 Apr 1;40(10):1133-1134. doi: 10.1200/JCO.22.00063. Epub 2022 Feb 16. J Clin Oncol. 2022. PMID: 35171688 No abstract available.
-
A Bit More Here and a Little Less There: The Trials (and Tribulations) of Adjuvant and Neoadjuvant Head and Neck Studies in 2021.Int J Radiat Oncol Biol Phys. 2022 Jun 1;113(2):243-251. doi: 10.1016/j.ijrobp.2022.02.016. Int J Radiat Oncol Biol Phys. 2022. PMID: 35569469 No abstract available.
References
-
- D'Souza G, Kreimer AR, Viscidi R, et al. : Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 356: 1944–19562007 - PubMed
-
- O'Sullivan B, Huang SH, Su J, et al. : Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): A multicentre cohort study. Lancet Oncol 17: 440–4512016 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- UG1 CA233331/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- UG1 CA233329/CA/NCI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U24 CA196172/CA/NCI NIH HHS/United States
- UG1 CA233337/CA/NCI NIH HHS/United States
- UG1 CA232760/CA/NCI NIH HHS/United States
- UG1 CA233196/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- UG1 CA233184/CA/NCI NIH HHS/United States
- UG1 CA233247/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- UG1 CA189953/CA/NCI NIH HHS/United States
- UG1 CA233328/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

