Early administration of remdesivir to COVID-19 patients associates with higher recovery rate and lower need for ICU admission: A retrospective cohort study
- PMID: 34699552
- PMCID: PMC8547637
- DOI: 10.1371/journal.pone.0258643
Early administration of remdesivir to COVID-19 patients associates with higher recovery rate and lower need for ICU admission: A retrospective cohort study
Abstract
Objectives: Remdesivir is one of the most widely recommended and used medications for COVID-19 treatment. However, different outcomes have been reported for hospitalized patients with COVID-19 treated with remdesivir. Specifically, the effect of the timing of remdesivir initiation (from patient's symptom onset) on clinical outcomes in COVID-19 patients has not been investigated.
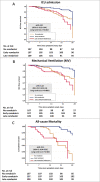
Methods: This is a retrospective cohort study of patients hospitalized with COVID-19 and treated with or without remdisivir. The primary outcome was patient's recovery rate, defined as clinical improvement and patient's discharge by day 14 of symptom onset. The secondary outcome was the need for intensive care unit (ICU) admission, mechanical ventilation, and mortality within 28 days of patient's symptom onset.
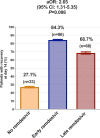
Results: Out of 323 hospitalized adults with COVID-19, 107 (33.1%) received no remdesivir during their hospital stay, 107 (33.1%) received remdesivir early within 7 days of the symptom onset, and 109 (33.7%) received it at 8 days or later of symptom onset. At day 14 following symptom onset, higher proportion of patients recovered in the early remdesivir compared to the late remdesivir cohort, or patients who did not receive remdesivir (adjusted odds ratio, aOR, 2.65; 95% confidence interval [CI], 1.31 to 5.35). Moreover, early administration of remdesivir was associated with lower admission to intensive care unit (adjusted hazard ratio [aHR], 0.31; 95% CI, 0.15 to 0.64), less need for mechanical ventilation (aHR, 0.22; 95% CI, 0.10 to 0.51), and lower mortality at 28 days (aHR, 0.15; 95% CI, 0.04 to 0.53), as compared to the late remdesivir cohort or patients who did not receive remdesivir.
Conclusion: Early administration of remdesivir within 7 days of symptom onset is associated with less need for mechanical ventilation and lower 28-days mortality.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, et al.. Repurposed Antiviral Drugs for Covid-19—Interim WHO Solidarity Trial Results. N Engl J Med. 2021;384(6):497–511. Epub 2020/12/03. doi: 10.1056/NEJMoa2023184 ; PubMed Central PMCID: PMC7727327. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

