Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL
- PMID: 34699592
- PMCID: PMC10652916
- DOI: 10.1182/blood.2021011895
Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL
Abstract
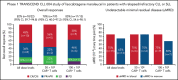
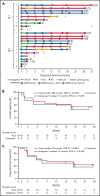
Bruton tyrosine kinase inhibitors (BTKi) and venetoclax are currently used to treat newly diagnosed and relapsed/refractory chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). However, most patients eventually develop resistance to these therapies, underscoring the need for effective new therapies. We report results of the phase 1 dose-escalation portion of the multicenter, open-label, phase 1/2 TRANSCEND CLL 004 (NCT03331198) study of lisocabtagene maraleucel (liso-cel), an autologous CD19-directed chimeric antigen receptor (CAR) T-cell therapy, in patients with relapsed/refractory CLL/SLL. Patients with standard- or high-risk features treated with ≥3 or ≥2 prior therapies, respectively, including a BTKi, received liso-cel at 1 of 2 dose levels (50 × 106 or 100 × 106 CAR+ T cells). Primary objectives included safety and determining recommended dose; antitumor activity by 2018 International Workshop on CLL guidelines was exploratory. Minimal residual disease (MRD) was assessed in blood and marrow. Twenty-three of 25 enrolled patients received liso-cel and were evaluable for safety. Patients had a median of 4 (range, 2-11) prior therapies (100% had ibrutinib; 65% had venetoclax) and 83% had high-risk features including mutated TP53 and del(17p). Seventy-four percent of patients had cytokine release syndrome (9% grade 3) and 39% had neurological events (22% grade 3/4). Of 22 efficacy-evaluable patients, 82% and 45% achieved overall and complete responses, respectively. Of 20 MRD-evaluable patients, 75% and 65% achieved undetectable MRD in blood and marrow, respectively. Safety and efficacy were similar between dose levels. The phase 2 portion of the study is ongoing at 100 × 106 CAR+ T cells. This trial was registered at clinicaltrials.gov as NCT03331198.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures



Comment in
-
Do CARs finally hit the CLL road?Blood. 2022 Mar 24;139(12):1775-1776. doi: 10.1182/blood.2021014492. Blood. 2022. PMID: 35323881 No abstract available.
References
-
- Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-Rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–1120. - PubMed
-
- Chisti MM. Chronic lymphocytic leukemia (CLL) guidelines. https://emedicine.medscape.com/article/199313-guidelines
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

