Allergic reactions to coronavirus disease 2019 vaccines and addressing vaccine hesitancy: Northwell Health experience
- PMID: 34699968
- PMCID: PMC8542398
- DOI: 10.1016/j.anai.2021.10.019
Allergic reactions to coronavirus disease 2019 vaccines and addressing vaccine hesitancy: Northwell Health experience
Abstract
Background: Allergic and nonallergic adverse reactions have been reported with global coronavirus disease 2019 (COVID-19) vaccination. It was previously hypothesized that polyethylene glycol (PEG) may be responsible for anaphylactic reactions to messenger RNA (mRNA) COVID-19 vaccines.
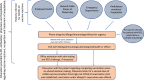
Objective: To report the workflow established at our institution, types, and frequency of adverse reactions to mRNA COVID-19 vaccines in patients presenting for allergy evaluation.
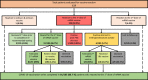
Methods: A COVID-19 vaccine adverse reaction registry was established. We used PEG prick skin testing, followed by PEG challenges in selected cases, to ensure PEG tolerance and encourage completion of COVID-19 vaccination series.
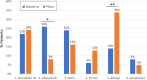
Results: A total of 113 patients were included. Most vaccine reactions (86.7%) occurred in women. Anaphylaxis occurred only in women, all of which had a history of allergic disease and two-thirds had asthma. Anaphylaxis rate was 40.6 cases per million. None of the anaphylactic cases developed hypotension, required intubation, or required hospital admission. Systemic allergic symptoms, not fulfilling anaphylaxis criteria, were significantly more common in Pfizer-BioNTech than Moderna-vaccinated patients (P = .02). We observed a higher incidence of dermatologic nonurticarial reactions in men (P = .004). Among first-dose reactors, 86.7% received and tolerated the second dose. We observed a high rate of false-positive intradermal skin test results and frequent subjective symptoms with oral PEG challenge.
Conclusion: Intradermal PEG testing has limited utility in evaluating anaphylaxis to mRNA vaccines. Most severe postvaccination allergic symptoms are not caused by hypersensitivity to PEG. Most people with reaction to the initial mRNA vaccine can be safely revaccinated. Patients with anaphylaxis to COVID-19 vaccines benefit from physician-observed vaccination.
Copyright © 2021 American College of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

