Influenza A Virus (H1N1) Infection Induces Microglial Activation and Temporal Dysbalance in Glutamatergic Synaptic Transmission
- PMID: 34700379
- PMCID: PMC8546584
- DOI: 10.1128/mBio.01776-21
Influenza A Virus (H1N1) Infection Induces Microglial Activation and Temporal Dysbalance in Glutamatergic Synaptic Transmission
Abstract
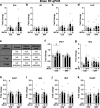
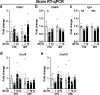
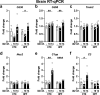
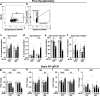
Influenza A virus (IAV) causes respiratory tract disease and is responsible for seasonal and reoccurring epidemics affecting all age groups. Next to typical disease symptoms, such as fever and fatigue, IAV infection has been associated with behavioral alterations presumably contributing to the development of major depression. Previous experiments using IAV/H1N1 infection models have shown impaired hippocampal neuronal morphology and cognitive abilities, but the underlying pathways have not been fully described. In this study, we demonstrate that infection with a low-dose non-neurotrophic H1N1 strain of IAV causes ample peripheral immune response followed by a temporary blood-brain barrier disturbance. Although histological examination did not reveal obvious pathological processes in the brains of IAV-infected mice, detailed multidimensional flow cytometric characterization of immune cells uncovered subtle alterations in the activation status of microglial cells. More specifically, we detected an altered expression pattern of major histocompatibility complex classes I and II, CD80, and F4/80 accompanied by elevated mRNA levels of CD36, CD68, C1QA, and C3, suggesting evolved synaptic pruning. To closer evaluate how these profound changes affect synaptic balance, we established a highly sensitive multiplex flow cytometry-based approach called flow synaptometry. The introduction of this novel technique enabled us to simultaneously quantify the abundance of pre- and postsynapses from distinct brain regions. Our data reveal a significant reduction of VGLUT1 in excitatory presynaptic terminals in the cortex and hippocampus, identifying a subtle dysbalance in glutamatergic synapse transmission upon H1N1 infection in mice. In conclusion, our results highlight the consequences of systemic IAV-triggered inflammation on the central nervous system and the induction and progression of neuronal alterations. IMPORTANCE Influenza A virus (IAV) causes mainly respiratory tract disease with fever and fatigue but is also associated with behavioral alterations in humans. Here, we demonstrate that infection with a low-dose non-neurotrophic H1N1 strain of IAV causes peripheral immune response followed by a temporary blood-brain barrier disturbance. Characterization of immune cells uncovered subtle alterations in the activation status of microglia cells that might reshape neuronal synapses. We established a highly sensitive multiplex flow cytometry-based approach called flow synaptometry to more closely study the synapses. Thus, we detected a specific dysbalance in glutamatergic synapse transmission upon H1N1 infection in mice. In conclusion, our results highlight the consequences of systemic IAV-triggered inflammation on the central nervous system and the induction and progression of neuronal alterations.
Keywords: flow synaptometry; glutamatergic synapse transmission; influenza; influenza A virus; interorgan communication; microglia; microglial activation; neuronal synapses; synaptosomes.
Figures




References
-
- Centers for Disease Control and Prevention. 2019. Seasonal influenza (flu): background and epidemiology. https://www.cdc.gov/flu/professionals/acip/background-epidemiology.htm.
-
- European Centre for Disease Prevention and Control. 2020. European Centre for Disease Prevention and Control Factsheet about seasonal influenza. https://www.ecdc.europa.eu/en/seasonal-influenza/facts/factsheet. Accessed 12 April 2021.
-
- European Centre for Disease Prevention and Control. 2020. Seasonal influenza 2019-2020. In ECDC (ed), Annual epidemiological report for 2019. European Centre for Disease Prevention and Control, Stockholm, Sweden.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

