Alcohol and the brain: from genes to circuits
- PMID: 34702580
- PMCID: PMC8616825
- DOI: 10.1016/j.tins.2021.09.006
Alcohol and the brain: from genes to circuits
Abstract
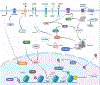
Alcohol use produces wide-ranging and diverse effects on the central nervous system. It influences intracellular signaling mechanisms, leading to changes in gene expression, chromatin remodeling, and translation. As a result of these molecular alterations, alcohol affects the activity of neuronal circuits. Together, these mechanisms produce long-lasting cellular adaptations in the brain that in turn can drive the development and maintenance of alcohol use disorder (AUD). We provide an update on alcohol research, focusing on multiple levels of alcohol-induced adaptations, from intracellular changes to changes in neural circuits. A better understanding of how alcohol affects these diverse and interlinked mechanisms may lead to the identification of novel therapeutic targets and to the development of much-needed novel and efficacious treatment options.
Keywords: alcohol; central nervous system; epigenetics; intracellular signaling; neural circuits.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

