Monoclonal antibody-mediated neutralization of SARS-CoV-2 in an IRF9-deficient child
- PMID: 34702736
- PMCID: PMC8609338
- DOI: 10.1073/pnas.2114390118
Monoclonal antibody-mediated neutralization of SARS-CoV-2 in an IRF9-deficient child
Abstract
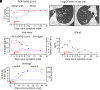
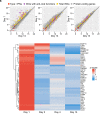
We describe an unvaccinated child at risk for life-threatening COVID-19 due to an inherited deficiency of IRF9, which governs ISGF-3-dependent responses to type I and III interferons (IFN). She was admitted, with a high nasal SARS-CoV-2 load on day 1 of upper respiratory tract infection. She was viremic on day 2 and received casirivimab and imdevimab. Her clinical manifestations and viremia disappeared on days 3 and 4, respectively. Circulating SARS-CoV-2 virus induced the expression of IFN-stimulated genes in leukocytes on day 1, whereas the secretion of blood type I IFNs, which peaked on day 4, did not. Antibody-mediated SARS-CoV-2 neutralization is, therefore, sufficient to overcome a deficiency of antiviral IFNs.
Keywords: COVID-19; RNA-seq; SARS-CoV-2; inherited primary immunodeficiency; interferon.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
References
-
- Brodin P., Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 27, 28–33 (2021). - PubMed
-
- Zhang Q., et al. ., COVID-STORM Clinicians; COVID Clinicians; Imagine COVID Group; French COVID Cohort Study Group; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort; NIAID-USUHS/TAGC COVID Immunity Group, Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 370, eabd4570 (2020). - PMC - PubMed
-
- Asano T., et al. ., COVID Human Genetic Effort; COVID-STORM Clinicians; COVID Clinicians; Imagine COVID Group; French COVID Cohort Study Group; CoV-Contact Cohort; Amsterdam UMC Covid-; Biobank; NIAID-USUHS COVID Study Group, X-linked recessive TLR7 deficiency in ∼1% of men under 60 years old with life-threatening COVID-19. Sci. Immunol. 6, eabl4348 (2021). - PMC - PubMed
-
- Bastard P., et al. ., HGID Lab; NIAID-USUHS Immune Response to COVID Group; COVID Clinicians; COVID-STORM Clinicians; Imagine COVID Group; French COVID Cohort Study Group; Milieu Intérieur Consortium; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort, Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370, eabd4585 (2020). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

