Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration
- PMID: 34702754
- PMCID: PMC8546498
- DOI: 10.1136/bmj.n2233
Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration
Abstract
Mendelian randomisation (MR) studies allow a better understanding of the causal effects of modifiable exposures on health outcomes, but the published evidence is often hampered by inadequate reporting. Reporting guidelines help authors effectively communicate all critical information about what was done and what was found. STROBE-MR (strengthening the reporting of observational studies in epidemiology using mendelian randomisation) assists authors in reporting their MR research clearly and transparently. Adopting STROBE-MR should help readers, reviewers, and journal editors evaluate the quality of published MR studies. This article explains the 20 items of the STROBE-MR checklist, along with their meaning and rationale, using terms defined in a glossary. Examples of transparent reporting are used for each item to illustrate best practices.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the SNSF, NIHR Biomedical Research Centre at University Hospitals Bristol, Weston NHS Foundation Trust, and University of Bristol for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; EWL (head of research at The BMJ) played no part in the peer review or decision making of this paper at the editorial level, and contributed solely as an author; no other relationships or activities that could appear to have influenced the submitted work. Provenance and peer review: Not commissioned; externally peer reviewed
Figures
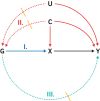
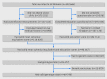
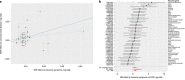
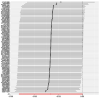
References
Publication types
MeSH terms
Grants and funding
- MC_UU_00011/2/MRC_/Medical Research Council/United Kingdom
- 19169/CRUK_/Cancer Research UK/United Kingdom
- R01 CA222147/CA/NCI NIH HHS/United States
- MC_UU_00011/7/MRC_/Medical Research Council/United Kingdom
- MC_UU_00011/1/MRC_/Medical Research Council/United Kingdom
- MR/M005070/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00011/6/MRC_/Medical Research Council/United Kingdom
- 001/WHO_/World Health Organization/International
- 29019/CRUK_/Cancer Research UK/United Kingdom
- MC_UU_00011/5/MRC_/Medical Research Council/United Kingdom
- MC_UU_00006/1/MRC_/Medical Research Council/United Kingdom
