Evaluation of a saliva molecular point of care for the detection of SARS-CoV-2 in ambulatory care
- PMID: 34702867
- PMCID: PMC8548486
- DOI: 10.1038/s41598-021-00560-8
Evaluation of a saliva molecular point of care for the detection of SARS-CoV-2 in ambulatory care
Abstract
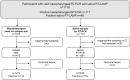
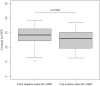
Rapid identification of SARS-CoV-2-infected individuals is a cornerstone for the control of virus spread. The sensitivity of SARS-CoV-2 RNA detection by RT-PCR is similar in saliva and nasopharyngeal swabs. Rapid molecular point-of-care tests in saliva could facilitate, broaden and speed up the diagnosis. We conducted a prospective study in two community COVID-19 screening centers to evaluate the performances of a CE-marked RT-LAMP assay (EasyCoV) designed for the detection of SARS-CoV2 RNA from fresh saliva samples, compared to nasopharyngeal RT-PCR, to saliva RT-PCR and to nasopharyngeal antigen testing. Overall, 117 of the 1718 participants (7%) tested positive with nasopharyngeal RT-PCR. Compared to nasopharyngeal RT-PCR, the sensitivity and specificity of the RT-LAMP assay in saliva were 34% and 97%, respectively. The Ct values of nasopharyngeal RT-PCR were significantly lower in the 40 true positive subjects with saliva RT-LAMP (Ct 25.9) than in the 48 false negative subjects with saliva RT-LAMP (Ct 28.4) (p = 0.028). Considering six alternate criteria for reference tests, including saliva RT-PCR and nasopharyngeal antigen, the sensitivity of saliva RT-LAMP ranged between 27 and 44%. The detection of SARS-CoV-2 in crude saliva samples with an RT-LAMP assay had a lower sensitivity than nasopharyngeal RT-PCR, saliva RT-PCR and nasopharyngeal antigen testing.Registration number: NCT04578509.
© 2021. The Author(s).
Conflict of interest statement
All authors and persons listed in the Acknowledgments declare no conflict of interest for the submitted work. Outside the submitted work, Solen Kernéis reports consulting fees, a research grant, honoraria for a lecture and travel expenses from bioMérieux (in 2018–2019); Jerome LeGoff reports consulting fees from bioMérieux and Roche Molecular (in 2018–2019); Constance Delaugerre reports to be member of a scientific board for MSD and Gilead ViiV, and a research grant from Gilead ViiV.
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

