Preventing adolescent synaptic pruning in mouse prelimbic cortex via local knockdown of α4βδ GABAA receptors increases anxiety response in adulthood
- PMID: 34702942
- PMCID: PMC8548505
- DOI: 10.1038/s41598-021-99965-8
Preventing adolescent synaptic pruning in mouse prelimbic cortex via local knockdown of α4βδ GABAA receptors increases anxiety response in adulthood
Abstract
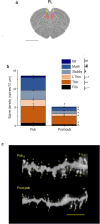
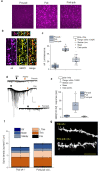
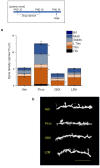
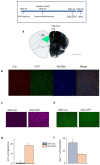
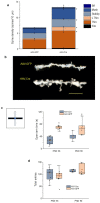
Anxiety is increasingly reported, especially in adolescent females. The etiology is largely unknown, which limits effective treatment. Layer 5 prelimbic cortex (L5PL) increases anxiety responses but undergoes adolescent synaptic pruning, raising the question of the impact of pruning on anxiety. Here we show that preventing L5PL pruning increases anxiety in response to an aversive event in adolescent and adult female mice. Spine density of Golgi-stained neurons decreased ~ 63% from puberty (~ PND35, vaginal opening) to post-puberty (PND56, P < 0.0001). Expression of α4βδ GABAA receptors (GABARs) transiently increased tenfold in L5PL at puberty (P < 0.00001), but decreased post-pubertally. Both global and local knockdown of these receptors during puberty prevented pruning, increasing spine density post-pubertally (P < 0.0001), an effect reversed by blocking NMDA receptors (NMDARs). Pubertal expression of the NMDAR-dependent spine protein kalirin7 decreased (50%, P < 0.0001), an effect prevented by α4 knock-out, suggesting that α4βδ-induced reductions in kalirin7 underlie pruning. Increased spine density due to local α4 knockdown at puberty decreased open arm time on the elevated plus maze post-pubertally (62%, P < 0.0001) in response to an aversive stimulus, suggesting that increases in L5PL synapses increase anxiety responses. These findings suggest that prelimbic synaptic pruning is necessary to limit anxiety in adulthood and may suggest novel therapies.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
α4βδ GABAA Receptors Trigger Synaptic Pruning and Reduce Dendritic Length of Female Mouse CA3 Hippocampal Pyramidal Cells at Puberty.Neuroscience. 2019 Feb 1;398:23-36. doi: 10.1016/j.neuroscience.2018.11.032. Epub 2018 Nov 26. Neuroscience. 2019. PMID: 30496825 Free PMC article.
-
α4βδ GABAA receptors reduce dendritic spine density in CA1 hippocampus and impair relearning ability of adolescent female mice: Effects of a GABA agonist and a stress steroid.Neuroscience. 2017 Apr 7;347:22-35. doi: 10.1016/j.neuroscience.2017.01.051. Epub 2017 Feb 9. Neuroscience. 2017. PMID: 28189613 Free PMC article.
-
Synaptic pruning in the female hippocampus is triggered at puberty by extrasynaptic GABAA receptors on dendritic spines.Elife. 2016 May 2;5:e15106. doi: 10.7554/eLife.15106. Elife. 2016. PMID: 27136678 Free PMC article.
-
α4βδ GABAA receptors and tonic inhibitory current during adolescence: effects on mood and synaptic plasticity.Front Neural Circuits. 2013 Sep 3;7:135. doi: 10.3389/fncir.2013.00135. eCollection 2013. Front Neural Circuits. 2013. PMID: 24027497 Free PMC article. Review.
-
The influence of stress at puberty on mood and learning: role of the α4βδ GABAA receptor.Neuroscience. 2013 Sep 26;249:192-213. doi: 10.1016/j.neuroscience.2012.09.065. Epub 2012 Oct 16. Neuroscience. 2013. PMID: 23079628 Free PMC article. Review.
Cited by
-
Sex differences in motor learning flexibility are accompanied by sex differences in mushroom spine pruning of the mouse primary motor cortex during adolescence.Front Neurosci. 2024 Jul 8;18:1420309. doi: 10.3389/fnins.2024.1420309. eCollection 2024. Front Neurosci. 2024. PMID: 39040633 Free PMC article.
-
Retrieval and savings of contextual fear memories across an extended retention interval in juvenile and adult male and female rats.Behav Neurosci. 2023 Dec;137(6):339-346. doi: 10.1037/bne0000569. Epub 2023 Oct 30. Behav Neurosci. 2023. PMID: 37902699 Free PMC article.
-
Abnormal Expression of Synaptic and Extrasynaptic GABAA Receptor Subunits in the Dystrophin-Deficient mdx Mouse.Int J Mol Sci. 2022 Oct 20;23(20):12617. doi: 10.3390/ijms232012617. Int J Mol Sci. 2022. PMID: 36293496 Free PMC article.
-
Morphine exposure during adolescence induces enduring social changes dependent on adolescent stage of exposure, sex, and social test.bioRxiv [Preprint]. 2023 Apr 22:2023.04.21.537856. doi: 10.1101/2023.04.21.537856. bioRxiv. 2023. Update in: Behav Neurosci. 2024 Feb;138(1):59-71. doi: 10.1037/bne0000567. PMID: 37131669 Free PMC article. Updated. Preprint.
References
-
- National College Health Assessment (2008–2019). https://www.acha.org/NCHA/ACHA-NCHA_Data/Publications_and_Reports. Accessed Mar 2021
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

