Mesenchymal stem cell treatment improves outcome of COVID-19 patients via multiple immunomodulatory mechanisms
- PMID: 34702946
- PMCID: PMC8546390
- DOI: 10.1038/s41422-021-00573-y
Mesenchymal stem cell treatment improves outcome of COVID-19 patients via multiple immunomodulatory mechanisms
Abstract
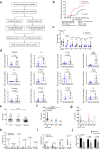
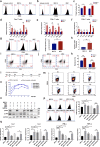
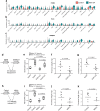
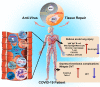
The infusion of coronavirus disease 2019 (COVID-19) patients with mesenchymal stem cells (MSCs) potentially improves clinical symptoms, but the underlying mechanism remains unclear. We conducted a randomized, single-blind, placebo-controlled (29 patients/group) phase II clinical trial to validate previous findings and explore the potential mechanisms. Patients treated with umbilical cord-derived MSCs exhibited a shorter hospital stay (P = 0.0198) and less time required for symptoms remission (P = 0.0194) than those who received placebo. Based on chest images, both severe and critical patients treated with MSCs showed improvement by day 7 (P = 0.0099) and day 21 (P = 0.0084). MSC-treated patients had fewer adverse events. MSC infusion reduced the levels of C-reactive protein, proinflammatory cytokines, and neutrophil extracellular traps (NETs) and promoted the maintenance of SARS-CoV-2-specific antibodies. To explore how MSCs modulate the immune system, we employed single-cell RNA sequencing analysis on peripheral blood. Our analysis identified a novel subpopulation of VNN2+ hematopoietic stem/progenitor-like (HSPC-like) cells expressing CSF3R and PTPRE that were mobilized following MSC infusion. Genes encoding chemotaxis factors - CX3CR1 and L-selectin - were upregulated in various immune cells. MSC treatment also regulated B cell subsets and increased the expression of costimulatory CD28 in T cells in vivo and in vitro. In addition, an in vivo mouse study confirmed that MSCs suppressed NET release and reduced venous thrombosis by upregulating kindlin-3 signaling. Together, our results underscore the role of MSCs in improving COVID-19 patient outcomes via maintenance of immune homeostasis.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
Treatment of COVID-19 with MSCs: how does it work?Sci China Life Sci. 2022 Apr;65(4):849-850. doi: 10.1007/s11427-021-2022-5. Epub 2021 Nov 5. Sci China Life Sci. 2022. PMID: 34755253 Free PMC article. No abstract available.
References
-
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;10:1001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

