A subpopulation of arenavirus nucleoprotein localizes to mitochondria
- PMID: 34702948
- PMCID: PMC8548533
- DOI: 10.1038/s41598-021-99887-5
A subpopulation of arenavirus nucleoprotein localizes to mitochondria
Abstract
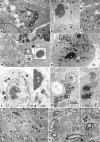
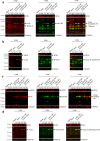
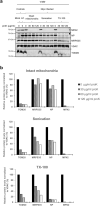
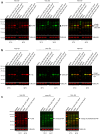
Viruses need cells for their replication and, therefore, ways to hijack cellular functions. Mitochondria play fundamental roles within the cell in metabolism, immunity and regulation of homeostasis due to which some viruses aim to alter mitochondrial functions. Herein we show that the nucleoprotein (NP) of arenaviruses enters the mitochondria of infected cells, affecting the mitochondrial morphology. Reptarenaviruses cause boid inclusion body disease (BIBD) that is characterized, especially in boas, by the formation of cytoplasmic inclusion bodies (IBs) comprising reptarenavirus NP within the infected cells. We initiated this study after observing electron-dense material reminiscent of IBs within the mitochondria of reptarenavirus infected boid cell cultures in an ultrastructural study. We employed immuno-electron microscopy to confirm that the mitochondrial inclusions indeed contain reptarenavirus NP. Mutations to a putative N-terminal mitochondrial targeting signal (MTS), identified via software predictions in both mamm- and reptarenavirus NPs, did not affect the mitochondrial localization of NP, suggesting that it occurs independently of MTS. In support of MTS-independent translocation, we did not detect cleavage of the putative MTSs of arenavirus NPs in reptilian or mammalian cells. Furthermore, in vitro translated NPs could not enter isolated mitochondria, suggesting that the translocation requires cellular factors or conditions. Our findings suggest that MTS-independent mitochondrial translocation of NP is a shared feature among arenaviruses. We speculate that by targeting the mitochondria arenaviruses aim to alter mitochondrial metabolism and homeostasis or affect the cellular defense.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Identification of Reptarenaviruses, Hartmaniviruses, and a Novel Chuvirus in Captive Native Brazilian Boa Constrictors with Boid Inclusion Body Disease.J Virol. 2020 May 18;94(11):e00001-20. doi: 10.1128/JVI.00001-20. Print 2020 May 18. J Virol. 2020. PMID: 32238580 Free PMC article.
-
Reptarenavirus S Segment RNA Levels Correlate with the Presence of Inclusion Bodies and the Number of L Segments in Snakes with Reptarenavirus Infection-Lessons Learned from a Large Breeding Colony.Microbiol Spectr. 2023 Jun 15;11(3):e0506522. doi: 10.1128/spectrum.05065-22. Epub 2023 May 22. Microbiol Spectr. 2023. PMID: 37212675 Free PMC article.
-
Replication of boid inclusion body disease-associated arenaviruses is temperature sensitive in both boid and mammalian cells.J Virol. 2015 Jan 15;89(2):1119-28. doi: 10.1128/JVI.03119-14. Epub 2014 Nov 5. J Virol. 2015. PMID: 25378485 Free PMC article.
-
Transcription and replication mechanisms of Bunyaviridae and Arenaviridae L proteins.Virus Res. 2017 Apr 15;234:118-134. doi: 10.1016/j.virusres.2017.01.018. Epub 2017 Jan 27. Virus Res. 2017. PMID: 28137457 Free PMC article. Review.
-
Brothers in Arms: Structure, Assembly and Function of Arenaviridae Nucleoprotein.Viruses. 2020 Jul 17;12(7):772. doi: 10.3390/v12070772. Viruses. 2020. PMID: 32708976 Free PMC article. Review.
Cited by
-
Boid Inclusion Body Disease Is Also a Disease of Wild Boa Constrictors.Microbiol Spectr. 2022 Oct 26;10(5):e0170522. doi: 10.1128/spectrum.01705-22. Epub 2022 Sep 12. Microbiol Spectr. 2022. PMID: 36094085 Free PMC article.
-
Experimental Reptarenavirus Infection of Boa constrictor and Python regius.J Virol. 2021 Mar 10;95(7):e01968-20. doi: 10.1128/JVI.01968-20. Epub 2021 Jan 13. J Virol. 2021. PMID: 33441344 Free PMC article.
-
A Multiplex RT-PCR Method for the Detection of Reptarenavirus Infection.Viruses. 2023 Nov 25;15(12):2313. doi: 10.3390/v15122313. Viruses. 2023. PMID: 38140554 Free PMC article.
-
Persistent Reptarenavirus and Hartmanivirus Infection in Cultured Boid Cells.Microbiol Spectr. 2022 Aug 31;10(4):e0158522. doi: 10.1128/spectrum.01585-22. Epub 2022 Jul 7. Microbiol Spectr. 2022. PMID: 35862992 Free PMC article.
-
Temperature affects reptarenavirus growth in a permissive host-derived in vitro model.J Gen Virol. 2025 Apr;106(4):002100. doi: 10.1099/jgv.0.002100. J Gen Virol. 2025. PMID: 40299760 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

