Bmal1 in the striatum influences alcohol intake in a sexually dimorphic manner
- PMID: 34702951
- PMCID: PMC8548330
- DOI: 10.1038/s42003-021-02715-9
Bmal1 in the striatum influences alcohol intake in a sexually dimorphic manner
Abstract
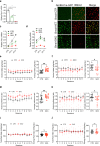
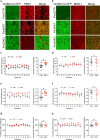
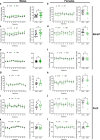
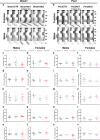
Alcohol consumption has been strongly associated with circadian clock gene expression in mammals. Analysis of clock genes revealed a potential role of Bmal1 in the control of alcohol drinking behavior. However, a causal role of Bmal1 and neural pathways through which it may influence alcohol intake have not yet been established. Here we show that selective ablation of Bmal1 (Cre/loxP system) from medium spiny neurons of the striatum induces sexual dimorphic alterations in alcohol consumption in mice, resulting in augmentation of voluntary alcohol intake in males and repression of intake in females. Per2mRNA expression, quantified by qPCR, decreases in the striatum after the deletion of Bmal1. To address the possibility that the effect of striatal Bmal1 deletion on alcohol intake and preference involves changes in the local expression of Per2, voluntary alcohol intake (two-bottle, free-choice paradigm) was studied in mice with a selective ablation of Per2 from medium spiny neurons of the striatum. Striatal ablation of Per2 increases voluntary alcohol intake in males but has no effect in females. Striatal Bmal1 and Per2 expression thus may contribute to the propensity to consume alcohol in a sex -specific manner in mice.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Characterization of Affective Behaviors and Motor Functions in Mice With a Striatal-Specific Deletion of Bmal1 and Per2.Front Physiol. 2022 Jun 8;13:922080. doi: 10.3389/fphys.2022.922080. eCollection 2022. Front Physiol. 2022. PMID: 35755440 Free PMC article.
-
Sexually dimorphic role of circadian clock genes in alcohol drinking behavior.Psychopharmacology (Berl). 2023 Mar;240(3):431-440. doi: 10.1007/s00213-022-06247-w. Epub 2022 Oct 3. Psychopharmacology (Berl). 2023. PMID: 36184679 Review.
-
Mapping the co-localization of the circadian proteins PER2 and BMAL1 with enkephalin and substance P throughout the rodent forebrain.PLoS One. 2017 Apr 19;12(4):e0176279. doi: 10.1371/journal.pone.0176279. eCollection 2017. PLoS One. 2017. PMID: 28423013 Free PMC article.
-
Growth Hormone Pulses and Liver Gene Expression Are Differentially Regulated by the Circadian Clock Gene Bmal1.Endocrinology. 2021 Apr 1;162(4):bqab023. doi: 10.1210/endocr/bqab023. Endocrinology. 2021. PMID: 33539533 Free PMC article.
-
Adenosinergic regulation of striatal clock gene expression and ethanol intake during constant light.Neuropsychopharmacology. 2014 Sep;39(10):2432-40. doi: 10.1038/npp.2014.94. Epub 2014 Apr 23. Neuropsychopharmacology. 2014. PMID: 24755889 Free PMC article.
Cited by
-
Haploinsufficiency of a Circadian Clock Gene Bmal1 (Arntl or Mop3) Causes Brain-Wide mTOR Hyperactivation and Autism-like Behavioral Phenotypes in Mice.Int J Mol Sci. 2022 Jun 5;23(11):6317. doi: 10.3390/ijms23116317. Int J Mol Sci. 2022. PMID: 35682995 Free PMC article.
-
Core clock gene, Bmal1, is required for optimal second-level interval production.Anim Cells Syst (Seoul). 2023 Dec 14;27(1):425-435. doi: 10.1080/19768354.2023.2290827. eCollection 2023. Anim Cells Syst (Seoul). 2023. PMID: 38125761 Free PMC article.
-
Bmal1 Modulates Striatal cAMP Signaling and Motor Learning.J Neurosci. 2025 Jul 9;45(28):e0474242025. doi: 10.1523/JNEUROSCI.0474-24.2025. J Neurosci. 2025. PMID: 40473476
-
Characterization of Affective Behaviors and Motor Functions in Mice With a Striatal-Specific Deletion of Bmal1 and Per2.Front Physiol. 2022 Jun 8;13:922080. doi: 10.3389/fphys.2022.922080. eCollection 2022. Front Physiol. 2022. PMID: 35755440 Free PMC article.
-
Antisense-Induced Downregulation of Clock Genes in the Shell Region of the Nucleus Accumbens Reduces Binge Drinking in Mice.Alcohol Clin Exp Res. 2021 Mar;45(3):530-542. doi: 10.1111/acer.14549. Epub 2021 Feb 19. Alcohol Clin Exp Res. 2021. PMID: 33606281 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

