Multi PCET in symmetrically substituted benzimidazoles
- PMID: 34703552
- PMCID: PMC8494046
- DOI: 10.1039/d1sc03782j
Multi PCET in symmetrically substituted benzimidazoles
Abstract
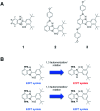
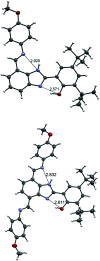
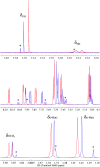
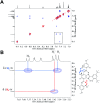
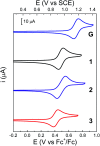
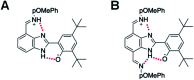
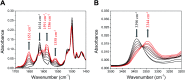
Proton-coupled electron transfer (PCET) reactions depend on the hydrogen-bond connectivity between sites of proton donors and acceptors. The 2-(2'-hydroxyphenyl) benzimidazole (BIP) based systems, which mimic the natural TyrZ-His190 pair of Photosystem II, have been useful for understanding the associated PCET process triggered by one-electron oxidation of the phenol. Substitution of the benzimidazole by an appropriate terminal proton acceptor (TPA) group allows for two-proton translocations. However, the prototropic properties of substituted benzimidazole rings and rotation around the bond linking the phenol and the benzimidazole can lead to isomers that interrupt the intramolecular hydrogen-bonded network and thereby prevent a second proton translocation. Herein, a strategic symmetrization of a benzimidazole based system with two identical TPAs yields an uninterrupted network of intramolecular hydrogen bonds regardless of the isomeric form. NMR data confirms the presence of a single isomeric form in the disubstituted system but not in the monosubstituted system in certain solvents. Infrared spectroelectrochemistry demonstrates a two-proton transfer process associated with the oxidation of the phenol occurring at a lower redox potential in the disubstituted system relative to its monosubstituted analogue. Computational studies support these findings and show that the disubstituted system stabilizes the oxidized two-proton transfer product through the formation of a bifurcated hydrogen bond. Considering the prototropic properties of the benzimidazole heterocycle in the context of multiple PCET will improve the next generation of novel, bioinspired constructs built by concatenated units of benzimidazoles, thus allowing proton translocations at nanoscale length.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
LinkOut - more resources
Full Text Sources

