A Systematic Review of Tafamidis in Patients With Transthyretin Amyloid Cardiomyopathy
- PMID: 34703707
- PMCID: PMC8541744
- DOI: 10.7759/cureus.18221
A Systematic Review of Tafamidis in Patients With Transthyretin Amyloid Cardiomyopathy
Abstract
Transthyretin amyloid cardiomyopathy disease burden is increasing daily due to advancements in diagnostic and imaging modalities in the modern world. Tafamidis is one of many therapeutic options. The main objective of this review is to study the role of Tafamidis in slowing the progression of transthyretin cardiomyopathy (TTR-CM) by analyzing randomized controlled trials (RCTs) and non-RCTs of Tafamidis. We searched for published papers of Tafamidis in the English language in electronic databases like Google Scholar, PubMed, Cochrane Library, and PubMed Central. We imported the resulting articles from our search to Mendeley software. Four reviewers removed the duplicates and performed title and abstract screening of the articles. The same reviewers obtained the full-text of relevant articles and did full-text screening based on eligibility criteria. Finally, five reviewers performed a quality assessment of RCTs using the Cochrane risk of bias assessment and of non-RCTs by a checklist prepared by Downs and Black. Any disagreements about any process were resolved by a discussion with other authors. One RCT and five non-RCTs of Tafamidis were included in this systematic review. From the non-RCTs, stability was observed in different parameters like echocardiographic findings, cardiac biomarkers, and ECG in patients with transthyretin cardiomyopathy during the study duration with Tafamidis. ATTR-ACT (Tafamidis in Transthyretin Cardiomyopathy Clinical Trial) trial demonstrated reduction of cardiovascular events and all-cause mortality in the Tafamidis group in comparison to placebo. In both RCT and non-RCTs, Tafamidis was established as a safe and tolerable drug for patients with TTR-CM. Our study proved the role of Tafamidis in reducing cardiovascular events, all-cause mortality, and the progression of cardiac disease in TTR-CM patients. In addition to five non-RCTs, current evidence is based on the findings of only one RCT of Tafamidis. Hence, evidence from additional RCTs is required to strongly support the stability of parameters like echocardiographic findings, cardiac biomarkers, and ECG with Tafamidis use.
Keywords: amyloid; cardiac biomarkers; cardiomyopathies; echocardiography; heart failure; outcomes; randomized controlled trial; tafamidis; transthyretin amyloid cardiomyopathy; ttr-stabilizing drug.
Copyright © 2021, Singh et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
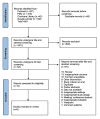
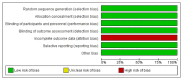
Figures
References
-
- A primer of amyloid nomenclature. Westermark P, Benson MD, Buxbaum JN, et al. Amyloid. 2007;14:179–183. - PubMed
-
- AL (light-chain) cardiac amyloidosis: a review of diagnosis and therapy. Falk RH, Alexander KM, Liao R, Dorbala S. J Am Coll Cardiol. 2016;68:1323–1341. - PubMed
-
- Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. Grogan M, Scott CG, Kyle RA, et al. J Am Coll Cardiol. 2016;68:1014–1020. - PubMed
-
- Progression of transthyretin (TTR) amyloidosis in donors and recipients after domino liver transplantation—a prospective single-center cohort study. Vollmar J, Schmid JC, Hoppe-Lotichius M, et al. Transpl Int. 2018;31:1207–1215. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
