Emerging Roles of Epigenetics in the Control of Reproductive Function: Focus on Central Neuroendocrine Mechanisms
- PMID: 34703958
- PMCID: PMC8533971
- DOI: 10.1210/jendso/bvab152
Emerging Roles of Epigenetics in the Control of Reproductive Function: Focus on Central Neuroendocrine Mechanisms
Abstract
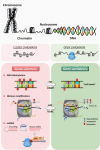
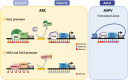
Reproduction is an essential function for perpetuation of the species. As such, it is controlled by sophisticated regulatory mechanisms that allow a perfect match between environmental conditions and internal cues to ensure adequate pubertal maturation and achievement of reproductive capacity. Besides classical genetic regulatory events, mounting evidence has documented that different epigenetic mechanisms operate at different levels of the reproductive axis to finely tune the development and function of this complex neuroendocrine system along the lifespan. In this mini-review, we summarize recent evidence on the role of epigenetics in the control of reproduction, with special focus on the modulation of the central components of this axis. Particular attention will be paid to the epigenetic control of puberty and Kiss1 neurons because major developments have taken place in this domain recently. In addition, the putative role of central epigenetic mechanisms in mediating the influence of nutritional and environmental cues on reproductive function will be discussed.
Keywords: GnRH; Kiss1; environmental cues; epigenetics; kisspeptins; nutrition; puberty; reproduction.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society.
Figures
References
-
- Tena-Sempere M, Huhtaniemi I. Gonadotropins and gonadotropin receptors. In: Fauser BCJM, ed. Reproductive Medicine - Molecular, Cellular and Genetic Fundamentals. New York: Parthenon Publishing; 2003:225-244.
-
- Avendaño MS, Vazquez MJ, Tena-Sempere M. Disentangling puberty: novel neuroendocrine pathways and mechanisms for the control of mammalian puberty. Hum Reprod Update. 2017;23(6):737-763. - PubMed
-
- Okamura H, Tsukamura H, Ohkura S, Uenoyama Y, Wakabayashi Y, Maeda K. Kisspeptin and GnRH pulse generation. Adv Exp Med Biol. 2013;784:297-323. - PubMed
-
- Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol. 2016;12(8):452-466. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
