Developing a Novel Measurement of Sleep in Rheumatoid Arthritis: Study Proposal for Approach and Considerations
- PMID: 34703974
- PMCID: PMC8460981
- DOI: 10.1159/000518024
Developing a Novel Measurement of Sleep in Rheumatoid Arthritis: Study Proposal for Approach and Considerations
Abstract
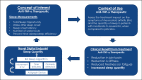
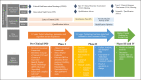
The development of novel digital endpoints (NDEs) using digital health technologies (DHTs) may provide opportunities to transform drug development. It requires a multidisciplinary, multi-study approach with strategic planning and a regulatory-guided pathway to achieve regulatory and clinical acceptance. Many NDEs have been explored; however, success has been limited. To advance industry use of NDEs to support drug development, we outline a theoretical, methodological study as a use-case proposal to describe the process and considerations when developing and obtaining regulatory acceptance for an NDE to assess sleep in patients with rheumatoid arthritis (RA). RA patients often suffer joint pain, fatigue, and sleep disturbances (SDs). Although many researchers have investigated the mobility of joint functions using wearable technologies, the research of SD in RA has been limited due to the availability of suitable technologies. We proposed measuring the improvement of sleep as the novel endpoint for an anti-TNF therapy and described the meaningfulness of the measure, considerations of tool selection, and the design of clinical validation. The recommendations from the FDA patient-focused drug development guidance, the Clinical Trials Transformation Initiative (CTTI) pathway for developing novel endpoints from DHTs, and the V3 framework developed by the Digital Medicine Society (DiMe) have been incorporated in the proposal. Regulatory strategy and engagement pathways are also discussed.
Keywords: Hypothetical study; Novel digital endpoint; Validation framework.
Copyright © 2021 by S. Karger AG, Basel.
Conflict of interest statement
The authors declare no conflicts of interest. However, all authors are employees and/or stockholders of the companies with which they are affiliated.
Figures

References
-
- Clinical Trials Transformation Initiative Novel endpoints project. 2017. Available from: https://www.ctti-clinicaltrials.org/projects/novel-endpoints.
-
- Digital Medicine Society Library of digital endpoints. 2020. Available from: https://www.dimesociety.org/index.php/knowledge-center/dime-generated-co....
-
- Nathan SD, Flaherty KR, Glassberg MK, Raghu G, Swigris J, Alvarez R, et al. A randomized, double-blind, placebo-controlled study of pulsed, inhaled nitric oxide in subjects at risk of pulmonary hypertension associated with pulmonary fibrosis. Chest. 2020;158:637–45. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

