BRAF V600 Mutation Detection in Plasma Cell-Free DNA: NCCTG N0879 (Alliance)
- PMID: 34703985
- PMCID: PMC8526905
- DOI: 10.1016/j.mayocpiqo.2021.05.003
BRAF V600 Mutation Detection in Plasma Cell-Free DNA: NCCTG N0879 (Alliance)
Abstract
Objective: To evaluate the prognostic significance of detectable circulating cell-free DNA (cfDNA) BRAF V600E/K mutations in patients with advanced melanoma enrolled in a clinical trial without BRAF-targeted therapy.
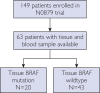
Patients and methods: BRAF V600E/K mutation status was determined on archived tissue and pretreatment stored plasma from 149 patients with unresectable stage IV melanoma who were enrolled between May 5, 2010 and May 2, 2014 in the North Central Cancer Treatment Group/Alliance N0879 randomized phase 2 clinical trial. Results were reported as presence or absence of cfDNA BRAF V600E/K detection of assay vs tissue. Progression-free survival (PFS) and overall survival (OS) were assessed for patients with and without detectable BRAF mutation.
Results: In total, 63 of 149 (42.3%) patients had BRAF V600E/K results for tissue and blood, and 20 of 63 (31.7%) patients had tissue-diagnosed mutant BRAF. Of these, 11 of 20 (55.0%) patients had detectable plasma cfDNA BRAF. Among patients with tissue-mutant BRAF V600E/K, PFS and OS were shorter for those with corresponding cfDNA mutations (PFS, 5.8 vs 12.0 months; P=.051; OS, 9.2 vs 27.1 months; P=.054). Our assay demonstrated sensitivity of 55% (95% CI, 0.322 to 0.768), specificity of 97.7% (95% CI, 0.932 to 1.000), positive predictive value of 91.7% (95% CI, 0.760 to 1.000), and negative predictive value of 82.4% (95% CI, 0.719 to 0.928).
Conclusion: In advanced melanoma, detectable cfDNA BRAF V600E/K mutation is present in about half the patients with stage IV with BRAF-mutant melanoma tumor tissue and appears to confer a poorer prognosis when detectable. Given the poorer prognosis, cfDNA can be used to risk-stratify patients with metastatic melanoma in practice or clinical trials.Trial Registration: clinicaltrials.gov Identifier: NCT00976573.
Keywords: FFPE, formalin-fixed paraffin-embedded; HR, hazard ratio; LDH, lactate dehydrogenase; MAPK, mitogen-activated protein kinase; NA, not available; NCCTG, North Central Cancer Treatment Group; NPV, negative predictive value; OS, overall survival; PFS, progression-free survival; PPV, positive predictive value; cfDNA, cell-free DNA; ddPCR, digital droplet polymerase chain reaction.
© 2021 THE AUTHORS.
Figures
References
-
- Atkinson V. Recent advances in malignant melanoma. Intern Med J. 2017;47(10):1114–1121. - PubMed
-
- Sanmamed M.F., Fernández-Landázuri S., Rodríguez C. Quantitative cell-free circulating BRAFV600E mutation analysis by use of droplet digital PCR in the follow-up of patients with melanoma being treated with BRAF inhibitors. Clin Chem. 2015;61(1):297–304. - PubMed
-
- Davies H., Bignell G.R., Cox C. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. - PubMed
-
- Wan P.T., Garnett M.J., Roe S.M. Cancer Genome Project. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116(6):855–867. - PubMed
-
- Janku F., Huang H.J., Claes B. BRAF mutation testing in cell-free DNA from the plasma of patients with advanced cancers using a rapid, automated molecular diagnostics system. Mol Cancer Ther. 2016;15(6):1397–1404. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials