A cell permeable bimane-constrained PCNA-interacting peptide
- PMID: 34704055
- PMCID: PMC8496261
- DOI: 10.1039/d1cb00113b
A cell permeable bimane-constrained PCNA-interacting peptide
Abstract
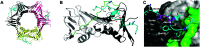
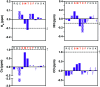
The human sliding clamp protein known as proliferating cell nuclear antigen (PCNA) orchestrates DNA-replication and -repair and as such is an ideal therapeutic target for proliferative diseases, including cancer. Peptides derived from the human p21 protein bind PCNA with high affinity via a 310-helical binding conformation and are known to shut down DNA-replication. Here, we present studies on short analogues of p21 peptides (143-151) conformationally constrained with a covalent linker between i, i + 4 separated cysteine residues at positions 145 and 149 to access peptidomimetics that target PCNA. The resulting macrocycles bind PCNA with K D values ranging from 570 nM to 3.86 μM, with the bimane-constrained peptide 7 proving the most potent. Subsequent X-ray crystallography and computational modelling studies of the macrocyclic peptides bound to PCNA indicated only the high-affinity peptide 7 adopted the classical 310-helical binding conformation. This suggests the 310-helical conformation is critical to high affinity PCNA binding, however NMR secondary shift analysis of peptide 7 revealed this secondary structure was not well-defined in solution. Peptide 7 is cell permeable and localised to the cell cytosol of breast cancer cells (MDA-MB-468), revealed by confocal microscopy showing blue fluorescence of the bimane linker. The inherent fluorescence of the bimane moiety present in peptide 7 allowed it to be directly imaged in the cell uptake assay, without attachment of an auxiliary fluorescent tag. This highlights a significant benefit of using a bimane constraint to access conformationally constrained macrocyclic peptides. This study identifies a small peptidomimetic that binds PCNA with higher affinity than previous reported p21 macrocycles, and is cell permeable, providing a significant advance toward development of a PCNA inhibitor for therapeutic applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

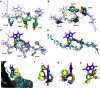


References
LinkOut - more resources
Full Text Sources
Miscellaneous

