Regioselective Magnesiation and Zincation Reactions of Aromatics and Heterocycles Triggered by Lewis Acids
- PMID: 34704653
- PMCID: PMC9300163
- DOI: 10.1002/chem.202103269
Regioselective Magnesiation and Zincation Reactions of Aromatics and Heterocycles Triggered by Lewis Acids
Abstract
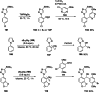
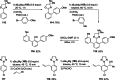
Mixed TMP-bases (TMP=2,2,6,6-tetramethylpiperidyl), such as TMPMgCl ⋅ LiCl, TMP2 Mg ⋅ 2LiCl, TMPZnCl ⋅ LiCl and TMP2 Zn ⋅ 2LiCl, are outstanding reagents for the metalation of functionalized aromatics and heterocycles. In the presence of Lewis acids, such as BF3 ⋅ OEt2 or MgCl2 , the metalation scope of such bases was dramatically increased, and regioselectivity switches were achieved in the presence or absence of these Lewis acids. Furthermore, highly reactive lithium bases, such as TMPLi or Cy2 NLi, are also compatible with various Lewis acids, such as MgCl2 ⋅ 2LiCl, ZnCl2 ⋅ 2LiCl or CuCN ⋅ 2LiCl. Performing such metalations in continuous flow using commercial setups permitted practical and convenient reaction conditions.
Keywords: frustrated Lewis pairs; magnesium; metalation reactions; regioselectivity; zinc.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

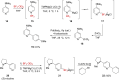


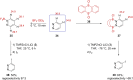

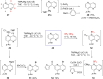

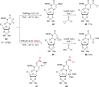








References
-
- P. Knochel, Handbook of Functionalized Organometallics, Wiley-VCH, Weinheim, 2005.
-
- None
-
- J. Clayden, Organolithiums: Selectivity for Synthesis, Vol. 23, Elsevier, 2002;
-
- R. Luisi, V. Capriati, Lithium Compounds in Organic Synthesis: From Fundamentals to Applications, John Wiley & Sons, 2014;
-
- Snieckus V., Chem. Rev. 1990, 90, 879–933;
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

