Deciphering the Role of Mucosal Immune Responses and the Cervicovaginal Microbiome in Resistance to HIV Infection in HIV-Exposed Seronegative (HESN) Women
- PMID: 34704803
- PMCID: PMC8549735
- DOI: 10.1128/Spectrum.00470-21
Deciphering the Role of Mucosal Immune Responses and the Cervicovaginal Microbiome in Resistance to HIV Infection in HIV-Exposed Seronegative (HESN) Women
Abstract
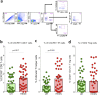
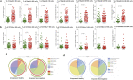
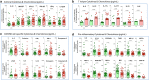
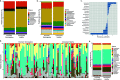
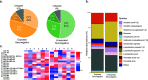
The female genital tract (FGT) is an important site of human immunodeficiency virus (HIV) infection. Discerning the nature of HIV-specific local immune responses is crucial for identifying correlates of protection in HIV-exposed seronegative (HESN) individuals. The present study involved a comprehensive analysis of soluble immune mediators, secretory immunoglobulins (sIg), natural killer (NK) cells, CXCR5+ CD8+ T cells, T follicular helper (Tfh) cells, and T regulatory cells (Tregs) in the vaginal mucosa as well as the nature and composition of the cervicovaginal microbiome in HESN women. We found significantly elevated antiviral cytokines, soluble immunoglobulins, and increased frequencies of activated NK cells, CXCR5+ CD8+ T cells, and Tfh cells in HESN females compared to HIV-unexposed healthy (UH) women. Analysis of the genital microbiome of HESN women revealed a greater bacterial diversity and increased abundance of Gardnerella spp. in the mucosa. The findings suggest that the female genital tract of HESN females represents a microenvironment equipped with innate immune factors, antiviral mediators, and critical T cell subsets that protect against HIV infection. IMPORTANCE The vast majority of human immunodeficiency virus (HIV) infections across the world occur via the sexual route. The genital tract mucosa is thus the primary site of HIV replication, and discerning the nature of HIV-specific immune responses in this compartment is crucial. The role of the innate immune system at the mucosal level in exposed seronegative individuals and other HIV controllers remains largely unexplored. This understanding can provide valuable insights to improve vaccine design. We investigated mucosal T follicular helper (Tfh) cells, CXCR5+ CD8+ T cells, natural killer (NK) cells subsets, soluble immune markers, and microbiome diversity in HIV-exposed seronegative (HESN) women. We found a significantly higher level of mucosal CXCR5+ CD8+ T cells, CD4+ Tfh cells, activated NK cell subsets, and antiviral immune cell mediators in HESN women. We also found a higher abundance of Gardnerella spp., microbiome dysbiosis, and decreased levels of inflammatory markers to be associated with reduced susceptibility to HIV infection. Our findings indicate that increased distribution of mucosal NK cells, CXCR5+ CD8+ T cells, Tfh cells, and soluble markers in HIV controllers with a highly diverse cervicovaginal microbiome could contribute effectively to protection against HIV infection. Overall, our findings imply that future vaccine design should emphasize inducing these highly functional cell types at the mucosal sites.
Keywords: B cells; CBA; CXCR5+ CD8+ cells; HESN; NK cells; TSCM cells; Tfh cells; cervicovaginal lavage; cervicovaginal microbiota; cytobrush; memory B cells.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

