Mapping SARS-CoV-2 Antibody Epitopes in COVID-19 Patients with a Multi-Coronavirus Protein Microarray
- PMID: 34704808
- PMCID: PMC8549749
- DOI: 10.1128/Spectrum.01416-21
Mapping SARS-CoV-2 Antibody Epitopes in COVID-19 Patients with a Multi-Coronavirus Protein Microarray
Abstract
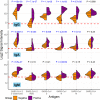
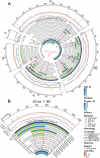
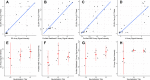
The rapid worldwide spread of SARS-CoV-2 has accelerated research and development for controlling the COVID-19 pandemic. A multi-coronavirus protein microarray was created containing full-length proteins, overlapping protein fragments of various lengths, and peptide libraries from SARS-CoV-2 and four other human coronaviruses. Sera from confirmed COVID-19 patients as well as unexposed individuals were applied to multicoronavirus arrays to identify specific antibody reactivity. High-level IgG, IgM, and IgA reactivity to structural proteins S, M, and N of SARS-CoV-2, as well as accessory proteins such as ORF3a and ORF7a, were observed that were specific to COVID-19 patients. Antibody reactivity against overlapping 100-, 50-, and 30-amino acid fragments of SARS-CoV-2 proteins was used to identify antigenic regions. Numerous proteins of SARS-CoV, Middle East respiratory syndrome coronavirus (MERS-CoV), and the endemic human coronaviruses HCoV-NL63 and HCoV-OC43 were also more reactive with IgG, IgM, and IgA in COVID-19 patient sera than in unexposed control sera, providing further evidence of immunologic cross-reactivity between these viruses. Whereas unexposed individuals had minimal reactivity against SARS-CoV-2 proteins that poorly correlated with reactivity against HCoV-NL63 and HCoV-OC43 S2 and N proteins, COVID-19 patient sera had higher correlation between SARS-CoV-2 and HCoV responses, suggesting that de novo antibodies against SARS-CoV-2 cross-react with HCoV epitopes. Array responses were compared with validated spike protein-specific IgG enzyme-linked immunosorbent assays (ELISAs), showing agreement between orthologous methods. SARS-CoV-2 microneutralization titers were low in the COVID-19 patient sera but correlated with array responses against S and N proteins. The multi-coronavirus protein microarray is a useful tool for mapping antibody reactivity in COVID-19 patients. IMPORTANCE With novel mutant SARS-CoV-2 variants of concern on the rise, knowledge of immune specificities against SARS-CoV-2 proteins is increasingly important for understanding the impact of structural changes in antibody-reactive protein epitopes on naturally acquired and vaccine-induced immunity, as well as broader topics of cross-reactivity and viral evolution. A multi-coronavirus protein microarray used to map the binding of COVID-19 patient antibodies to SARS-CoV-2 proteins and protein fragments as well as to the proteins of four other coronaviruses that infect humans has shown specific regions of SARS-CoV-2 proteins that are highly reactive with patient antibodies and revealed cross-reactivity of these antibodies with other human coronaviruses. These data and the multi-coronavirus protein microarray tool will help guide further studies of the antibody response to COVID-19 and to vaccination against this worldwide pandemic.
Keywords: COVID-19; HCoV; SARS-CoV-2; antibody binding sites.
Figures



References
-
- WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/director-general/speeches/detail/who-director-genera....
-
- Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, Cuomo-Dannenburg G, Thompson H, Walker PGT, Fu H, Dighe A, Griffin JT, Baguelin M, Bhatia S, Boonyasiri A, Cori A, Cucunubá Z, FitzJohn R, Gaythorpe K, Green W, Hamlet A, Hinsley W, Laydon D, Nedjati-Gilani G, Riley S, van Elsland S, Volz E, Wang H, Wang Y, Xi X, Donnelly CA, Ghani AC, Ferguson NM. 2020. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis 20:669–677. doi:10.1016/S1473-3099(20)30243-7. - DOI - PMC - PubMed
-
- Long Q-X, Liu B-Z, Deng H-J, Wu G-C, Deng K, Chen Y-K, Liao P, Qiu J-F, Lin Y, Cai X-F, Wang D-Q, Hu Y, Ren J-H, Tang N, Xu Y-Y, Yu L-H, Mo Z, Gong F, Zhang X-L, Tian W-G, Hu L, Zhang X-X, Xiang J-L, Du H-X, Liu H-W, Lang C-H, Luo X-H, Wu S-B, Cui X-P, Zhou Z, Zhu M-M, Wang J, Xue C-J, Li X-F, Wang L, Li Z-J, Wang K, Niu C-C, Yang Q-J, Tang X-J, Zhang Y, Liu X-M, Li J-J, Zhang D-C, Zhang F, Liu P, Yuan J, Li Q, Hu J-L, Chen J, et al. . 2020. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 26:845–848. doi:10.1038/s41591-020-0897-1. - DOI - PubMed
-
- Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, Qian S, Hong C, Wang F, Liu Y, Wang Z, He Q, Li Z, He B, Zhang T, Fu Y, Ge S, Liu L, Zhang J, Xia N, Zhang Z. 2020. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis 71:2027–2034. doi:10.1093/cid/ciaa344. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

