Metabolic regulation of RA macrophages is distinct from RA fibroblasts and blockade of glycolysis alleviates inflammatory phenotype in both cell types
- PMID: 34705053
- PMCID: PMC8739866
- DOI: 10.1007/s00018-021-03978-5
Metabolic regulation of RA macrophages is distinct from RA fibroblasts and blockade of glycolysis alleviates inflammatory phenotype in both cell types
Abstract
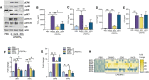
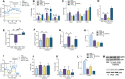
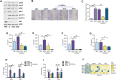
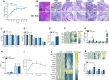
Recent studies have shown the significance of metabolic reprogramming in immune and stromal cell function. Yet, the metabolic reconfiguration of RA macrophages (MΦs) is incompletely understood during active disease and in crosstalk with other cell types in experimental arthritis. This study elucidates a distinct regulation of glycolysis and oxidative phosphorylation in RA MΦs compared to fibroblast (FLS), although PPP (Pentose Phosphate pathway) is similarly reconfigured in both cell types. 2-DG treatment showed a more robust impact on impairing the RA M1 MΦ-mediated inflammatory phenotype than IACS-010759 (IACS, complexli), by reversing ERK, AKT and STAT1 signaling, IRF8/3 transcription and CCL2 or CCL5 secretion. This broader inhibitory effect of 2-DG therapy on RA M1 MΦs was linked to dysregulation of glycolysis (GLUT1, PFKFB3, LDHA, lactate) and oxidative PPP (NADP conversion to NADPH), while both compounds were ineffective on oxidative phosphorylation. Distinctly, in RA FLS, 2-DG and IACS therapies constrained LPS/IFNγ-induced AKT and JNK signaling, IRF5/7 and fibrokine expression. Disruption of RA FLS metabolic rewiring by 2-DG or IACS therapy was accompanied by a reduction of glycolysis (HIF1α, PFKFB3) and suppression of citrate or succinate buildup. We found that 2-DG therapy mitigated CIA pathology by intercepting joint F480+iNOS+MΦ, Vimentin+ fibroblast and CD3+T cell trafficking along with downregulation of IRFs and glycolytic intermediates. Surprisingly, IACS treatment was inconsequential on CIA swelling, cell infiltration, M1 and Th1/Th17 cytokines (IFN-γ/IL-17) and joint glycolytic mediators. Collectively, our results indicate that blockade of glycolysis is more effective than inhibition of complex 1 in CIA, in part due to its effectiveness on the MΦ inflammatory phenotype.
Keywords: Glycolysis; Mitochondrial oxidative phosphorylation and CIA; RA FLS; RA macrophages.
© 2021. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors have declared that no commercial or financial conflict of interest exists.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

