Biosimilar-to-Biosimilar Switching: What is the Rationale and Current Experience?
- PMID: 34705255
- PMCID: PMC8578069
- DOI: 10.1007/s40265-021-01610-1
Biosimilar-to-Biosimilar Switching: What is the Rationale and Current Experience?
Abstract
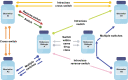
Over time, clinicians have become increasingly comfortable embracing the prescription of biosimilars-highly similar versions of innovator or reference biological agents-for their patients with inflammatory diseases. Although a switch from a reference product to a licensed biosimilar version (or vice versa) is a medical decision robustly supported by the stepwise accumulation of clinical trial evidence concerning comparable safety, immunogenicity, and efficacy between these products, a switch from one biosimilar to another biosimilar of the same reference product, or a cross-switch, is not. Similarity among biosimilars of a reference product is not a regulatory agency concern and therefore is unlikely to be investigated in randomized controlled trials in the foreseeable future. Yet in clinical practice, across a diverse range of patients, the option to cross-switch from one biosimilar to another can and does arise for valid reasons such as convenience or tolerability issues, or driven by third parties (e.g., payers). In the absence of clinical trial data, clinicians must attempt to objectively evaluate the emerging real-world cross-switching evidence within the context of what is known about the science underpinning a designation of biosimilar. That knowledge then needs to be integrated with what clinicians know about their patients and their disease on a case-by-case basis. This review aims to consolidate relevant emerging real-world data and other key information about biosimilar-to-biosimilar cross-switching for prescribing clinicians. In the absence of clear clinical guidelines addressing this topic at present, this review may serve to facilitate discretionary and educated treatment decision making.
© 2021. The Author(s).
Conflict of interest statement
BI, DA, and MM are full-time employees of and own stock or options in Pfizer. NI was a full-time employee of Pfizer at the time the manuscript was developed and owns stock or options in Pfizer. EM received or has pending grants from Roche, Pfizer, Bristol-Myers Squibb and Novartis received honorarium from Eli Lilly, Pfizer, GlaxoSmithKline, Roche, Sanofi, AstraZeneca, Sandoz, Amgen, Gemmene, and AbbVie provided writing assistance, medicines, equipment, or administrative support to Pfizer, AbbVie, and Roche, and received payment for lectures including service on speakers’ bureaus to Eli Lilly, Pfizer, GlaxoSmithKline, Roche, Sanofi, AstraZeneca, Sandoz, Amgen, Gema Biotech, and AbbVie. VFA has received grants to conduct trials on biosimilars from Boehringer Ingelheim, consulting fees related to advisory boards from Pfizer, Amgen, and Sandoz, and payment for lectures provided to Pfizer, Amgen, Sandoz, Celltrion, and Janssen. SD reports consultancy fees from AbbVie, Allergan, Amgen, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingleheim, Celgene, Celltrion, Eli Lilly, Enthera, Ferring Pharmaceuticals Inc., Gilead, Hospira, Inotrem, Janssen, Johnson & Johnson, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, TiGenix, UCB Inc., and Vifor. LP-B served as a speaker, consultant, and advisory member for AbbVie, Allergan, Alma, Amgen, Applied Molecular Transport, Arena, Biogen, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Celltrion, Enterome, Enthera, Ferring, Fresenius Kabi SwissBioSim GmbH, Genentech, Gilead, Hikma, Index Pharmaceuticals, Janssen, Eli Lilly, MSD, Mylan, Nestle, Norgine, Oppilan Pharma, OSE Immunotherapeutics, Pfizer, Pharmacosmos, Roche, Samsung Bioepis, Sandoz, Sterna, Sublimity Therapeutics, Takeda, Tillots, and Vifor, received grants from AbbVie, MSD, and Takeda, and owns stock options from CTMA.
Figures






References
-
- Lauret A, Molto A, Abitbol V, Gutermann L, Conort O, Chast F, et al. Effects of successive switches to different biosimilars infliximab on immunogenicity in chronic inflammatory diseases in daily clinical practice. Semin Arthritis Rheum. 2020;50(6):1449–1456. - PubMed
-
- Thomas L. Biosimilars: providing more treatment options. 2019. https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_7071.pdf. Accessed 5 Nov 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

