Small Molecule Control of Morpholino Antisense Oligonucleotide Function through Staudinger Reduction
- PMID: 34705461
- PMCID: PMC9014490
- DOI: 10.1021/jacs.1c08723
Small Molecule Control of Morpholino Antisense Oligonucleotide Function through Staudinger Reduction
Abstract
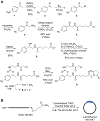
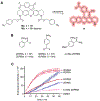
Conditionally activated, caged morpholino antisense agents (cMOs) are tools that enable the temporal and spatial investigation of gene expression, regulation, and function during embryonic development. Cyclic MOs are conformationally gated oligonucleotide analogs that do not block gene expression until they are linearized through the application of an external trigger, such as light or enzyme activity. Here, we describe the first examples of small molecule-responsive cMOs, which undergo rapid and efficient decaging via a Staudinger reduction. This is enabled by a highly flexible linker design that offers opportunities for the installation of chemically activated, self-immolative motifs. We synthesized cyclic cMOs against two distinct, developmentally relevant genes and demonstrated phosphine-triggered knockdown of gene expression in zebrafish embryos. This represents the first report of a small molecule-triggered antisense agent for gene knockdown, adding another bioorthogonal entry to the growing arsenal of gene knockdown tools.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Chakraborty S; Mehtab S; Krishnan Y The predictive power of synthetic nucleic acid technologies in RNA biology. Acc. Chem. Res 2014, 47 (6), 1710–9. - PubMed
-
- Setten RL; Rossi JJ; Han SP The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discovery 2019, 18 (6), 421–446. - PubMed
-
- Summerton JE Morpholino, siRNA, and S-DNA compared: impact of structure and mechanism of action on off-target effects and sequence specificity. Curr. Top. Med. Chem 2007, 7 (7), 651–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

