Antarctic Polyester Hydrolases Degrade Aliphatic and Aromatic Polyesters at Moderate Temperatures
- PMID: 34705547
- PMCID: PMC8752145
- DOI: 10.1128/AEM.01842-21
Antarctic Polyester Hydrolases Degrade Aliphatic and Aromatic Polyesters at Moderate Temperatures
Abstract
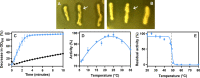
Polyethylene terephthalate (PET) is one of the most widely used synthetic plastics in the packaging industry, and consequently has become one of the main components of plastic waste found in the environment. However, several microorganisms have been described to encode enzymes that catalyze the depolymerization of PET. While most known PET hydrolases are thermophilic and require reaction temperatures between 60°C and 70°C for an efficient hydrolysis of PET, a partial hydrolysis of amorphous PET at lower temperatures by the polyester hydrolase IsPETase from the mesophilic bacterium Ideonella sakaiensis has also been reported. We show that polyester hydrolases from the Antarctic bacteria Moraxella sp. strain TA144 (Mors1) and Oleispira antarctica RB-8 (OaCut) were able to hydrolyze the aliphatic polyester polycaprolactone as well as the aromatic polyester PET at a reaction temperature of 25°C. Mors1 caused a weight loss of amorphous PET films and thus constitutes a PET-degrading psychrophilic enzyme. Comparative modeling of Mors1 showed that the amino acid composition of its active site resembled both thermophilic and mesophilic PET hydrolases. Lastly, bioinformatic analysis of Antarctic metagenomic samples demonstrated that members of the Moraxellaceae family carry candidate genes coding for further potential psychrophilic PET hydrolases. IMPORTANCE A myriad of consumer products contains polyethylene terephthalate (PET), a plastic that has accumulated as waste in the environment due to its long-term stability and poor waste management. One promising solution is the enzymatic biodegradation of PET, with most known enzymes only catalyzing this process at high temperatures. Here, we bioinformatically identified and biochemically characterized an enzyme from an Antarctic organism that degrades PET at 25°C with similar efficiency to the few PET-degrading enzymes active at moderate temperatures. Reasoning that Antarctica harbors other PET-degrading enzymes, we analyzed available data from Antarctic metagenomic samples and successfully identified other potential enzymes. Our findings contribute to increasing the repertoire of known PET-degrading enzymes that are currently being considered as biocatalysts for the biological recycling of plastic waste.
Keywords: Antarctica; Moraxella sp.; Oleispira antarctica; Polyethylene terephthalate (PET); plastic biodegradation; polyester hydrolases; psychrophilic enzymes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Scott G. 2006. Polymers in modern life, p 1–18. In Scott G (ed), Polymers and the environment. RSC Publishing, London, UK.
-
- Satsangi S. 2017. Polyethylene terephthalate (PET) market by application (beverages, sheet & films, consumer goods, food packaging, and others) and end-use industry (packaging, electrical & electronics, automotive, construction, and others): global opportunity analysis and industry forecast, 2017–2023. Big Market Research, Portland, OR, USA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

