Chronic Release of Tailless Phage Particles from Lactococcus lactis
- PMID: 34705552
- PMCID: PMC8752148
- DOI: 10.1128/AEM.01483-21
Chronic Release of Tailless Phage Particles from Lactococcus lactis
Abstract
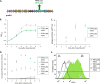
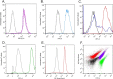
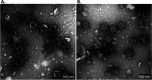
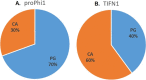
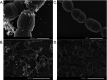
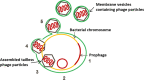
Lactococcus lactis strains residing in the microbial community of a complex dairy starter culture named "Ur" are hosts to prophages belonging to the family Siphoviridae. L. lactis strains (TIFN1 to TIFN7) showed detectable spontaneous phage production and release (109 to 1010 phage particles/ml) and up to 10-fold increases upon prophage induction, while in both cases we observed no obvious cell lysis typically described for the lytic life cycle of Siphoviridae phages. Intrigued by this phenomenon, we investigated the host-phage interaction using strain TIFN1 (harboring prophage proPhi1) as a representative. We confirmed that during the massive phage release, all bacterial cells remain viable. Further, by monitoring phage replication in vivo, using a green fluorescence protein reporter combined with flow cytometry, we demonstrated that the majority of the bacterial population (over 80%) is actively producing phage particles when induced with mitomycin C. The released tailless phage particles were found to be engulfed in lipid membranes, as evidenced by electron microscopy and lipid staining combined with chemical lipid analysis. Based on the collective observations, we propose a model of phage-host interaction in L. lactis TIFN1 where the phage particles are engulfed in membranes upon release, thereby leaving the producing host intact. Moreover, we discuss possible mechanisms of chronic, or nonlytic, release of LAB Siphoviridae phages and its impact on the bacterial host. IMPORTANCE In complex microbial consortia such as fermentation starters, bacteriophages can alter the dynamics and diversity of microbial communities. Bacteriophages infecting Lactococcus lactis are mostly studied for their detrimental impact on industrial dairy fermentation processes. In this study, we describe a novel form of phage-bacterium interaction in an L. lactis strain isolated from a complex dairy starter culture: when the prophages harbored in the L. lactis genome are activated, the phage particles are engulfed in lipid membranes upon release, leaving the producing host intact. Findings from this study provide additional insights into the diverse manners of phage-bacterium interactions and coevolution, which are essential for understanding the population dynamics in complex microbial communities like fermentation starters.
Keywords: Siphoviridae; dairy starter culture; lipid bilayer; membranes; nonlytic phage release.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

