Protective Efficacy of Gastrointestinal SARS-CoV-2 Delivery against Intranasal and Intratracheal SARS-CoV-2 Challenge in Rhesus Macaques
- PMID: 34705557
- PMCID: PMC8791250
- DOI: 10.1128/JVI.01599-21
Protective Efficacy of Gastrointestinal SARS-CoV-2 Delivery against Intranasal and Intratracheal SARS-CoV-2 Challenge in Rhesus Macaques
Abstract
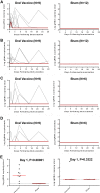
Live oral vaccines have been explored for their protective efficacy against respiratory viruses, particularly for adenovirus serotypes 4 and 7. The potential of a live oral vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), however, remains unclear. In this study, we assessed the immunogenicity of live SARS-CoV-2 delivered to the gastrointestinal tract in rhesus macaques and its protective efficacy against intranasal and intratracheal SARS-CoV-2 challenge. Postpyloric administration of SARS-CoV-2 by esophagogastroduodenoscopy resulted in limited virus replication in the gastrointestinal tract and minimal to no induction of mucosal antibody titers in rectal swabs, nasal swabs, and bronchoalveolar lavage fluid. Low levels of serum neutralizing antibodies were induced and correlated with modestly diminished viral loads in nasal swabs and bronchoalveolar lavage fluid following intranasal and intratracheal SARS-CoV-2 challenge. Overall, our data show that postpyloric inoculation of live SARS-CoV-2 is weakly immunogenic and confers partial protection against respiratory SARS-CoV-2 challenge in rhesus macaques. IMPORTANCE SARS-CoV-2 remains a global threat, despite the rapid deployment but limited coverage of multiple vaccines. Alternative vaccine strategies that have favorable manufacturing timelines, greater ease of distribution, and improved coverage may offer significant public health benefits, especially in resource-limited settings. Live oral vaccines have the potential to address some of these limitations; however, no studies have yet been conducted to assess the immunogenicity and protective efficacy of a live oral vaccine against SARS-CoV-2. Here, we report that oral administration of live SARS-CoV-2 in nonhuman primates may offer prophylactic benefits, but the formulation and route of administration will require further optimization.
Keywords: COVID-19; SARS-CoV-2; immunogenicity; live oral vaccine; protective efficacy.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

