B cell activating factor (BAFF) from neutrophils and dendritic cells is required for protective B cell responses against Salmonella typhimurium infection
- PMID: 34705890
- PMCID: PMC8550399
- DOI: 10.1371/journal.pone.0259158
B cell activating factor (BAFF) from neutrophils and dendritic cells is required for protective B cell responses against Salmonella typhimurium infection
Abstract
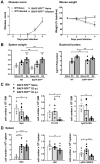
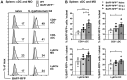
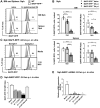
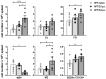
Mice lacking B cells are more susceptible to S. typhimurium infection. How B cells contribute to protective immunity against Salmonella and what signals drive their activation are still unclear. Neutrophils (Nphs), monocytes (MOs), and dendritic cells (DCs) are involved in early immune responses to control the initial replication of S. typhimurium. These cells can produce B cell activating factor (BAFF) required for mature B cell survival and may help regulate B cell responses during Salmonella infection. Using BAFF reporter mice (BAFF-RFP+/-), we discovered that an i.p. infection with a virulent strain of S. typhimurium increased BAFF expression in splenic conventional DCs (cDC) and inflammatory Ly6Chi MOs/DCs four days post-infection. S. typhimurium infection induced the release of BAFF from Nphs, a decrease of BAFF-RFP expression and expansion of BAFF-RFP+ Nphs in the spleen and peritoneal cavity. After S. typhimurium infection, serum BAFF levels and immature and mature B cell subsets and plasma cells increased substantially. Conditional knockout (cKO) mice lacking BAFF in either Nphs or cDCs compared to control Bafffl/fl mice had reduced up-regulation of systemic BAFF levels and reduced expansion of mature and germinal center B cell subsets after infection. Importantly, the cKO mice lacking BAFF from either Nphs or cDCs had impaired induction of Salmonella-specific IgM Abs, and were more susceptible to S. typhimurium infection. Thus, Nphs and cDCs are major cellular sources of BAFF driving B cell responses, required for mounting optimal protective immunity against lethal Salmonella infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
B cell-activating factor (BAFF) from dendritic cells, monocytes and neutrophils is required for B cell maturation and autoantibody production in SLE-like autoimmune disease.Front Immunol. 2023 Feb 27;14:1050528. doi: 10.3389/fimmu.2023.1050528. eCollection 2023. Front Immunol. 2023. PMID: 36923413 Free PMC article.
-
BAFF Produced by Neutrophils and Dendritic Cells Is Regulated Differently and Has Distinct Roles in Antibody Responses and Protective Immunity against West Nile Virus.J Immunol. 2020 Mar 15;204(6):1508-1520. doi: 10.4049/jimmunol.1901120. Epub 2020 Feb 7. J Immunol. 2020. PMID: 32034064 Free PMC article.
-
Early cellular responses to Salmonella infection: dendritic cells, monocytes, and more.Immunol Rev. 2008 Oct;225:140-62. doi: 10.1111/j.1600-065X.2008.00679.x. Immunol Rev. 2008. PMID: 18837781 Review.
-
Interleukin-33 Contributes Toward Loss of Tolerance by Promoting B-Cell-Activating Factor of the Tumor-Necrosis-Factor Family (BAFF)-Dependent Autoantibody Production.Front Immunol. 2018 Dec 6;9:2871. doi: 10.3389/fimmu.2018.02871. eCollection 2018. Front Immunol. 2018. PMID: 30574145 Free PMC article.
-
Mechanisms used by virulent Salmonella to impair dendritic cell function and evade adaptive immunity.Immunology. 2012 Sep;137(1):28-36. doi: 10.1111/j.1365-2567.2012.03614.x. Immunology. 2012. PMID: 22703384 Free PMC article. Review.
Cited by
-
Thymus antibody-secreting cells possess an interferon gene signature and are preferentially expanded in young female mice.iScience. 2023 Feb 15;26(3):106223. doi: 10.1016/j.isci.2023.106223. eCollection 2023 Mar 17. iScience. 2023. PMID: 36890795 Free PMC article.
-
Changes in monocyte subsets in volunteers who received an oral wild-type Salmonella Typhi challenge and reached typhoid diagnosis criteria.Front Immunol. 2024 Aug 27;15:1454857. doi: 10.3389/fimmu.2024.1454857. eCollection 2024. Front Immunol. 2024. PMID: 39263222 Free PMC article.
-
Type I interferon induced during chronic viral infection favors B-cell development in the thymus.Immunol Cell Biol. 2024 Oct;102(9):801-816. doi: 10.1111/imcb.12808. Epub 2024 Jul 15. Immunol Cell Biol. 2024. PMID: 39009814
-
B cell-activating factor (BAFF) from dendritic cells, monocytes and neutrophils is required for B cell maturation and autoantibody production in SLE-like autoimmune disease.Front Immunol. 2023 Feb 27;14:1050528. doi: 10.3389/fimmu.2023.1050528. eCollection 2023. Front Immunol. 2023. PMID: 36923413 Free PMC article.
-
A humanized mouse that mounts mature class-switched, hypermutated and neutralizing antibody responses.Nat Immunol. 2024 Aug;25(8):1489-1506. doi: 10.1038/s41590-024-01880-3. Epub 2024 Jun 25. Nat Immunol. 2024. PMID: 38918608 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

