Egg yolk immunoglobulin (IgY) targeting SARS-CoV-2 S1 as potential virus entry blocker
- PMID: 34706134
- PMCID: PMC8657347
- DOI: 10.1111/jam.15340
Egg yolk immunoglobulin (IgY) targeting SARS-CoV-2 S1 as potential virus entry blocker
Abstract
Aims: COVID-19 pandemic caused by SARS-CoV-2 has become a public health crisis worldwide. In this study, we aimed at demonstrating the neutralizing potential of the IgY produced after immunizing chicken with a recombinant SARS-CoV-2 spike protein S1 subunit.
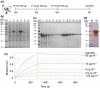
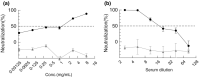
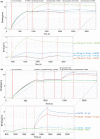
Methods and results: E. coli BL21 carrying plasmid pET28a-S1 was induced with IPTG for the expression of SARS-CoV-2 S1 protein. The recombinant His-tagged S1 was purified and verified by SDS-PAGE, Western blot and biolayer interferometry (BLI) assay. Then S1 protein emulsified with Freund's adjuvant was used to immunize layer chickens. Specific IgY against S1 (S1-IgY) produced from egg yolks of these chickens exhibited a high titer (1:25,600) and a strong binding affinity to S1 (KD = 318 nmol L-1 ). The neutralizing ability of S1-IgY was quantified by a SARS-CoV-2 pseudotyped virus-based neutralization assay with an IC50 value of 0.99 mg ml-1 . In addition, S1-IgY exhibited a strong ability in blocking the binding of SARS-CoV-2 S1 to hACE2, and it could partially compete with hACE2 for the binding sites on S1 by BLI assays.
Conclusions: We demonstrated here that after immunization of chickens with our recombinant S1 protein, IgY neutralizing antibodies were generated against the SARS-CoV-2 spike protein S1 subunit; therefore, showing the potential use of IgY to block the entry of this virus.
Significance and impact of the study: IgY targeting S1 subunit of SARS-CoV-2 could be a promising candidate for pre- and post-exposure prophylaxis or treatment of COVID-19. Administration of IgY-based oral preparation, oral or nasal spray may have profound implications for blocking SARS-CoV-2.
Keywords: COVID-19; S1; SARS-CoV-2; antibody; egg yolk immunoglobulin Y (IgY); hACE2.
© 2021 The Society for Applied Microbiology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Immunoglobulin yolk targeting spike 1, receptor binding domain of spike glycoprotein and nucleocapsid of SARS-CoV-2 blocking RBD-ACE2 binding interaction.Int Immunopharmacol. 2022 Nov;112:109280. doi: 10.1016/j.intimp.2022.109280. Epub 2022 Sep 28. Int Immunopharmacol. 2022. PMID: 36183680 Free PMC article.
-
Generation of Chicken IgY against SARS-COV-2 Spike Protein and Epitope Mapping.J Immunol Res. 2020 Oct 17;2020:9465398. doi: 10.1155/2020/9465398. eCollection 2020. J Immunol Res. 2020. PMID: 33134398 Free PMC article.
-
Chicken Egg Yolk Antibodies (IgYs) block the binding of multiple SARS-CoV-2 spike protein variants to human ACE2.Int Immunopharmacol. 2021 Jan;90:107172. doi: 10.1016/j.intimp.2020.107172. Epub 2020 Nov 3. Int Immunopharmacol. 2021. PMID: 33191178 Free PMC article.
-
An approach towards development of monoclonal IgY antibodies against SARS CoV-2 spike protein (S) using phage display method: A review.Int Immunopharmacol. 2020 Aug;85:106654. doi: 10.1016/j.intimp.2020.106654. Epub 2020 Jun 3. Int Immunopharmacol. 2020. PMID: 32512271 Free PMC article. Review.
-
Chicken IgY Antibodies Provide Mucosal Barrier against SARS-CoV-2 Virus and Other Pathogens.Isr Med Assoc J. 2021 Apr;23(4):208-211. Isr Med Assoc J. 2021. PMID: 33899350 Review.
Cited by
-
Polyclonal anti-whole cell IgY passive immunotherapy shields against P. aeruginosa-induced acute pneumonia and burn wound infections in murine models.Sci Rep. 2024 Jan 3;14(1):405. doi: 10.1038/s41598-023-50859-x. Sci Rep. 2024. PMID: 38172232 Free PMC article.
-
Are Hamsters a Suitable Model for Evaluating the Immunogenicity of RBD-Based Anti-COVID-19 Subunit Vaccines?Viruses. 2022 May 16;14(5):1060. doi: 10.3390/v14051060. Viruses. 2022. PMID: 35632800 Free PMC article.
-
Monoclonal IgY antibodies: advancements and limitations for immunodiagnosis and immunotherapy applications.Ther Adv Vaccines Immunother. 2024 Jul 25;12:25151355241264520. doi: 10.1177/25151355241264520. eCollection 2024. Ther Adv Vaccines Immunother. 2024. PMID: 39071998 Free PMC article. Review.
-
Use of Immunoglobulin Y Antibodies: Biosensor-based Diagnostic Systems and Prophylactic and Therapeutic Drug Delivery Systems for Viral Respiratory Diseases.Curr Top Med Chem. 2024;24(11):973-985. doi: 10.2174/0115680266289898240322073258. Curr Top Med Chem. 2024. PMID: 38561616 Review.
-
Antiviral Immunoglobulins of Chicken Egg Yolk for Potential Prevention of SARS-CoV-2 Infection.Viruses. 2022 Sep 26;14(10):2121. doi: 10.3390/v14102121. Viruses. 2022. PMID: 36298676 Free PMC article.
References
-
- Adachi, K. , Handharyani, E. , Sari, D.K. , Takama, K. , Fukuda, K. , Endo, I. et al. (2008) Development of neutralization antibodies against highly pathogenic H5N1 avian influenza virus using ostrich (Struthio camelus) yolk. Molecular Medicine Reports, 1, 203–209. - PubMed
-
- Akita, E.M. & Nakai, S. (1992) Immunoglobulins from egg yolk: isolation and purification. Journal of Food Science, 57(3), 629–634.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

