An active vesicle priming machinery suppresses axon regeneration upon adult CNS injury
- PMID: 34706221
- PMCID: PMC8730507
- DOI: 10.1016/j.neuron.2021.10.007
An active vesicle priming machinery suppresses axon regeneration upon adult CNS injury
Abstract
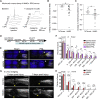
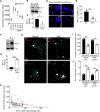
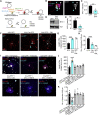
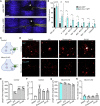
Axons in the adult mammalian central nervous system fail to regenerate after spinal cord injury. Neurons lose their capacity to regenerate during development, but the intracellular processes underlying this loss are unclear. We found that critical components of the presynaptic active zone prevent axon regeneration in adult mice. Transcriptomic analysis combined with live-cell imaging revealed that adult primary sensory neurons downregulate molecular constituents of the synapse as they acquire the ability to rapidly grow their axons. Pharmacogenetic reduction of neuronal excitability stimulated axon regeneration after adult spinal cord injury. Genetic gain- and loss-of-function experiments uncovered that essential synaptic vesicle priming proteins of the presynaptic active zone, but not clostridial-toxin-sensitive VAMP-family SNARE proteins, inhibit axon regeneration. Systemic administration of Baclofen reduced voltage-dependent Ca2+ influx in primary sensory neurons and promoted their regeneration after spinal cord injury. These findings indicate that functional presynaptic active zones constitute a major barrier to axon regeneration.
Keywords: Baclofen; Munc13; RIM1/2; active zone; axon injury; axon regeneration; spinal cord injury.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests. N.B. is a member of the Neuron advisory board.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

