Monoclonal antibodies protect aged rhesus macaques from SARS-CoV-2-induced immune activation and neuroinflammation
- PMID: 34706272
- PMCID: PMC8523485
- DOI: 10.1016/j.celrep.2021.109942
Monoclonal antibodies protect aged rhesus macaques from SARS-CoV-2-induced immune activation and neuroinflammation
Abstract
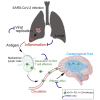
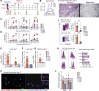
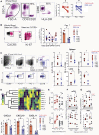
Anti-viral monoclonal antibody (mAb) treatments may provide immediate but short-term immunity from coronavirus disease 2019 (COVID-19) in high-risk populations, such as people with diabetes and the elderly; however, data on their efficacy in these populations are limited. We demonstrate that prophylactic mAb treatment blocks viral replication in both the upper and lower respiratory tracts in aged, type 2 diabetic rhesus macaques. mAb infusion dramatically curtails severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-mediated stimulation of interferon-induced chemokines and T cell activation, significantly reducing development of interstitial pneumonia. Furthermore, mAb infusion significantly dampens the greater than 3-fold increase in SARS-CoV-2-induced effector CD4 T cell influx into the cerebrospinal fluid. Our data show that neutralizing mAbs administered preventatively to high-risk populations may mitigate the adverse inflammatory consequences of SARS-CoV-2 exposure.
Keywords: NeuroCOVID, inflammation; SARS-CoV-2; cerebrospinal fluid; effector CD4 T cells; interstitial pneumonia; lymph node; neuroinflammation; pathogenesis; rhesus macaques.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Chang M.C., Hild S., Grieder F. Nonhuman primate models for SARS-CoV-2 research: Consider alternatives to macaques. Lab Anim. (NY) 2021;50:113–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

