Ionophore-mediated swelling of erythrocytes as a therapeutic mechanism in sickle cell disease
- PMID: 34706495
- PMCID: PMC9152977
- DOI: 10.3324/haematol.2021.278666
Ionophore-mediated swelling of erythrocytes as a therapeutic mechanism in sickle cell disease
Abstract
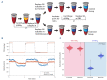
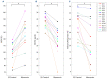
Sickle cell disease (SCD) is characterized by sickle hemoglobin (HbS) which polymerizes under deoxygenated conditions to form a stiff, sickled erythrocyte. The dehydration of sickle erythrocytes increases intracellular HbS concentration and the propensity of erythrocyte sickling. Prevention of this mechanism may provide a target for potential SCD therapy investigation. Ionophores such as monensin can increase erythrocyte sodium permeability by facilitating its transmembrane transport, leading to osmotic swelling of the erythrocyte and decreased hemoglobin concentration. In this study, we treated 13 blood samples from patients with SCD with 10 nM of monensin ex vivo. We measured changes in cell volume and hemoglobin concentration in response to monensin treatment, and we perfused treated blood samples through a microfluidic device that permits quantification of blood flow under controlled hypoxia. Monensin treatment led to increases in cell volume and reductions in hemoglobin concentration in most blood samples, though the degree of response varied across samples. Monensin-treated samples also demonstrated reduced blood flow impairment under hypoxic conditions relative to untreated controls. Moreover, there was a significant correlation between the improvement in blood flow and the decrease in hemoglobin concentration. Thus, our results demonstrate that a reduction in intracellular HbS concentration by osmotic swelling improves blood flow under hypoxic conditions. Although the toxicity of monensin will likely prevent it from being a viable clinical treatment, these results suggest that osmotic swelling should be investigated further as a potential mechanism for SCD therapy.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

