The Canadian prospective cohort study to understand progression in multiple sclerosis (CanProCo): rationale, aims, and study design
- PMID: 34706670
- PMCID: PMC8549411
- DOI: 10.1186/s12883-021-02447-7
The Canadian prospective cohort study to understand progression in multiple sclerosis (CanProCo): rationale, aims, and study design
Abstract
Background: Neurological disability progression occurs across the spectrum of people living with multiple sclerosis (MS). Although there are a handful of disease-modifying treatments approved for use in progressive phenotypes of MS, there are no treatments that substantially modify the course of clinical progression in MS. Characterizing the determinants of clinical progression can inform the development of novel therapeutic agents and treatment approaches that target progression in MS, which is one of the greatest unmet needs in clinical practice. Canada, having one of the world's highest rates of MS and a publicly-funded health care system, represents an optimal country to achieve in-depth analysis of progression. Accordingly, the overarching aim of the Canadian Prospective Cohort Study to Understand Progression in MS (CanProCo) is to evaluate a wide spectrum of factors associated with the clinical onset and rate of disease progression in MS, and to describe how these factors relate to one another to influence progression.
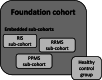
Methods: CanProCo is a prospective, observational cohort study with investigators specializing in epidemiology, neuroimaging, neuroimmunology, health services research and health economics. CanProCo's study design was approved by an international review panel, comprised of content experts and key stakeholders. One thousand individuals with radiologically-isolated syndrome, relapsing-remitting MS, and primary-progressive MS within 10-15 years of disease onset will be recruited from 5 academic MS centres in Canada. Participants will undergo detailed clinical evaluation annually over 5 years (including advanced, app-based clinical data collection). In a subset of participants within 5-10 years of disease onset (n = 500), blood, cerebrospinal fluid, and research MRIs will be collected allowing an integrated, in-depth evaluation of factors contributing to progression in MS from multiple perspectives. Factors of interest range from biological measures (e.g. single-cell RNA-sequencing), MRI-based microstructural assessment, participant characteristics (self-reported, performance-based, clinician-assessed, health-system based), and micro and macro-environmental factors.
Discussion: Halting the progression of MS remains a fundamental need to improve the lives of people living with MS. Achieving this requires leveraging transdisciplinary approaches to better characterize why clinical progression occurs. CanProCo is a pioneering multi-dimensional cohort study aiming to characterize these determinants to inform the development and implementation of efficacious and effective interventions.
Keywords: Biology; Canada; Cohort; Epidemiology; Health systems; Imaging; Multiple sclerosis; Neuroimmunology; Progression; Progressive MS; Prospective.
© 2021. The Author(s).
Conflict of interest statement
Jiwon Oh has received research funding from Biogen-Idec and Roche, and personal fees for consulting for Biogen-Idec and Roche.
Nathalie Arbour reports no competing interests.
Fabrizio Giuliani has received honoraria from Biogen, Novartis, Roche for industry-sponsored talks, and consulting fees from Biogen and Novartis. He is the recipient of research grants from Biogen and Roche.
Melanie Guenette reports no competing interests.
Shannon Kolind has received research funding from Roche.
Larry Lynd reports no competing interests.
Ruth Ann Marrie reports no competing interests.
Luanne M. Metz reports no competing interests.
Scott B. Patten reports no competing interests.
Alexandre Prat has received research support from Biogen and Roche; has received consulting fees from Biogen-Idec and Roche.
Alice Schabas has received personal fees for consulting for Biogen-Idec and Roche.
Penny Smyth has received unrestricted research support from Biogen-Idec; and has received consulting fees and honoraria for speaking activities from Biogen-Idec and Roche.
Roger Tam holds a grant from the Praxis Spinal Cord Institute for an unrelated educational program.
Anthony Traboulsee has received research support from Sanofi Genzyme and Roche; has received consulting fees from Sanofi Genzyme, Roche, Teva Neuroscience, Novartis, Biogen and EMD Serono; and has received honoraria for his involvement in speaker bureau activities for Sanofi Genzyme and Roche.
V. Wee Yong has received honoraria from Biogen, Novartis, Roche, Sanofi-Genzyme and Teva for industry-sponsored talks, and consulting fees from EMD Serono, Roche and Sanofi-Genzyme. He is the recipient of unrestricted educational grants from Biogen, EMD Serono, Novartis, Roche, Sanofi-Genzyme and Teva to support educational activities of the Alberta MS Network, which he directs.
Figures
Similar articles
-
The Canadian Prospective Cohort Study to understand progression in multiple sclerosis: baseline characteristics.Ther Adv Neurol Disord. 2024 Sep 12;17:17562864241273045. doi: 10.1177/17562864241273045. eCollection 2024. Ther Adv Neurol Disord. 2024. PMID: 39282637 Free PMC article.
-
The Cannabinoid Use in Progressive Inflammatory brain Disease (CUPID) trial: a randomised double-blind placebo-controlled parallel-group multicentre trial and economic evaluation of cannabinoids to slow progression in multiple sclerosis.Health Technol Assess. 2015 Feb;19(12):vii-viii, xxv-xxxi, 1-187. doi: 10.3310/hta19120. Health Technol Assess. 2015. PMID: 25676540 Free PMC article. Clinical Trial.
-
Glial and neuroaxonal biomarkers in a multiple sclerosis (MS) cohort.Hell J Nucl Med. 2019 Sep-Dec;22 Suppl 2:113-121. Hell J Nucl Med. 2019. PMID: 31802051
-
Approaches and challenges in the diagnosis and management of secondary progressive multiple sclerosis: A Central Eastern European perspective from healthcare professionals.Mult Scler Relat Disord. 2021 May;50:102778. doi: 10.1016/j.msard.2021.102778. Epub 2021 Jan 28. Mult Scler Relat Disord. 2021. PMID: 33592384 Review.
-
Diagnosis and treatment of progressive multiple sclerosis: A position paper.Eur J Neurol. 2023 Jan;30(1):9-21. doi: 10.1111/ene.15593. Epub 2022 Oct 25. Eur J Neurol. 2023. PMID: 36209464 Free PMC article. Review.
Cited by
-
The Canadian Prospective Cohort Study to understand progression in multiple sclerosis: baseline characteristics.Ther Adv Neurol Disord. 2024 Sep 12;17:17562864241273045. doi: 10.1177/17562864241273045. eCollection 2024. Ther Adv Neurol Disord. 2024. PMID: 39282637 Free PMC article.
-
Reproducible Spinal Cord Quantitative MRI Analysis with the Spinal Cord Toolbox.Magn Reson Med Sci. 2024 Jul 1;23(3):307-315. doi: 10.2463/mrms.rev.2023-0159. Epub 2024 Mar 12. Magn Reson Med Sci. 2024. PMID: 38479843 Free PMC article. Review.
-
Employment status, productivity loss, and associated factors among people with multiple sclerosis.Mult Scler. 2023 Jun;29(7):866-874. doi: 10.1177/13524585231164295. Epub 2023 Apr 15. Mult Scler. 2023. PMID: 37060245 Free PMC article.
-
Neuroimaging to monitor worsening of multiple sclerosis: advances supported by the grant for multiple sclerosis innovation.Front Neurol. 2023 Dec 1;14:1319869. doi: 10.3389/fneur.2023.1319869. eCollection 2023. Front Neurol. 2023. PMID: 38107636 Free PMC article. Review.
-
Use of smartphone-based remote assessments of multiple sclerosis in Floodlight Open, a global, prospective, open-access study.Sci Rep. 2024 Jan 2;14(1):122. doi: 10.1038/s41598-023-49299-4. Sci Rep. 2024. PMID: 38168498 Free PMC article.
References
-
- Federation MI. Atlas of MS [Available from: https://www.atlasofms.org/fact-sheet/canada.
-
- International Progressive MS Alliance [Available from: https://www.progressivemsalliance.org/.
-
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. - DOI - PubMed
-
- Kappos L, Wolinsky JS, Giovannoni G, Arnold DL, Wang Q, Bernasconi C, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132–1140. doi: 10.1001/jamaneurol.2020.1568. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical