Ado-trastuzumab for the treatment of metastatic HER2-positive breast cancer in patients previously treated with Pertuzumab
- PMID: 34706686
- PMCID: PMC8549287
- DOI: 10.1186/s12885-021-08894-2
Ado-trastuzumab for the treatment of metastatic HER2-positive breast cancer in patients previously treated with Pertuzumab
Abstract
Background: Docetaxel in combination with two HER2-directed therapies, trastuzumab and pertuzumab, is the current standard frontline therapy for patients with metastatic HER2-positive breast cancer. Ado-trastuzumab (T-DM1), an antibody-drug conjugate of trastuzumab and a cytotoxic microtubule-inhibitory agent, emtansine, is approved in patients that have progressed with prior trastuzumab-based therapy. However, the benefit of T-DM1 in patients previously treated with pertuzumab therapy for metastatic breast cancer remains unclear.
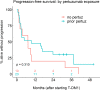
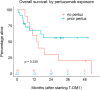
Methods: We identified thirty-three adults with metastatic HER2-positive breast cancer treated between March 2013 and July 2018 with T-DM1 either as subsequent therapy after progression on a pertuzumab-based regimen (i.e., "pertuzumab-pretreated") or without prior exposure to pertuzumab (i.e., "pertuzumab-naïve"). Collected data included patient demographics, treatment history, adverse events, and clinical outcomes. For both cohorts receiving T-DM1, the primary endpoint was PFS and secondary endpoints were overall survival (OS), overall response rate (ORR), clinical benefit rate (CBR), and T-DM1-related toxicity rate.
Results: Pertuzumab-pretreated patients (n = 23, with 21 evaluable for T-DM1 efficacy) had a median PFS of 9.5 months (95% CI: 2.9-NA), 1-year OS rate of 67.4% (95% CI: 50.0-90.9%) with an unreached median, ORR of 14.3% (95% CI: 3.0-36.3%), and CBR of 52.4% (95% CI: 29.8-74.3%), with none of these measures being statistically different than those estimated for the pertuzumab-naïve group (n = 10). Treatment with T-DM1 after prior pertuzumab exposure (median T-DM1 duration 2.9 months) resulted in no grade ≥ 3 adverse events.
Conclusions: In our cohort, prior exposure to pertuzumab did not significantly impact T-DM1's clinical efficacy or safety profile as second- or later-line therapy in patients with metastatic HER2-positive breast cancer.
Keywords: Ado-Trastuzumab; Breast Cancer; HER2; Pertuzumab; T-DM1.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane L, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF, American Society of Clinical Oncology., College of American Pathologists Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. - DOI - PubMed
-
- Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, Robert NJ, Silovski T, Gokmen E, von Minckwitz G, Ejlertsen B, Chia SKL, Mansi J, Barrios CH, Gnant M, Buyse M, Gore I, 2nd Smith J, Harker G, Masuda N, Petrakova K, Zotano AG, Iannotti N, Rodriguez G, Tassone P, Wong A, Bryce R, Ye Y, Yao B, Martin M, ExteNET Study Group Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncology. 2016;17(3):367–377. doi: 10.1016/S1470-2045(15)00551-3. - DOI - PubMed
-
- von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N, Clark E, Knott A, Lang I, Levy C, Yardley DA, Bines J, Gelber RD, Piccart M, Baselga J, APHINITY Steering Committee and Investigators Adjuvant Pertuzumab and Trastuzumab in early HER2-positive breast Cancer. N Engl J Med. 2017;377(2):122–131. doi: 10.1056/NEJMoa1703643. - DOI - PMC - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast Cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous