Crude extract and isolated bioactive compounds from Notholirion thomsonianum (Royale) Stapf as multitargets antidiabetic agents: in-vitro and molecular docking approaches
- PMID: 34706708
- PMCID: PMC8549260
- DOI: 10.1186/s12906-021-03443-7
Crude extract and isolated bioactive compounds from Notholirion thomsonianum (Royale) Stapf as multitargets antidiabetic agents: in-vitro and molecular docking approaches
Abstract
Background: Diabetes mellitus is a common disease effecting the lifestyles of majority world population. In this research work, we have embarked the potential role of crude extracts and isolated compounds of Notholirion thomsonianum for the management diabetes mellitus.
Methods: The crude extracts of N. thomsonianum were initially evaluated for α-glucosidase, α-amylase and antioxidant activities. The compounds were isolated from the activity based potent solvent fraction. The structures of isolated compounds were confirmed with NMR and MS analyses. The isolated compounds were tested for α-glucosidase, α-amylase, protein tyrosine phosphatase 1B (PTP1B) and DPPH activities. The molecular docking studies were carried out to find the binding interactions of isolated compounds for α-glucosidase, α-amylase and PTP1B.
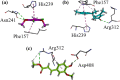
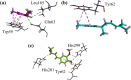
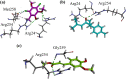
Results: Initially, we screened out crude extracts and subfractions of N. thomsonianum against different in-vitro targets. Among all, Nt.EtAc was observed a potent fraction among all giving IC50 values of 67, 70, < 0.1, 89 and 16 μg/mL against α-glucosidase, α-amylase, DPPH, ABTS and H2O2 respectively. Three compounds (Nt01, Nt02 and Nt03) were isolated from Nt.EtAc of N. thomsonianum. The isolated compounds Nt01, Nt02 and Nt03 exhibited IC50 values of 58.93, 114.93 and 19.54 μM against α-glucosidase, while 56.25, 96.54 and 24.39 μM against α-amylase respectively. Comparatively, the standard acarbose observed IC50 values were 10.60 and 12.71 μM against α-glucosidase, α-amylase respectively. In PTP1B assay, the compounds Nt01, Nt02 and Nt03 demonstrated IC50 values of 12.96, 36.22 and 3.57 μM in comparison to the standard ursolic acid (IC50 of 3.63 μM). The isolated compounds also gave overwhelming results in DPPH assay. Molecular docking based binding interactions for α-glucosidase, α-amylase and PTP1B were also encouraging.
Conclusions: In the light of current results, it is obvious that N. thomsonianum is potential medicinal plant for the treatment of hyperglycemia. Overall, Nt.EtAc was dominant fraction in all in-vitro activities. Three compounds Nt01, Nt02 and Nt03 were isolated from ethyl acetate fraction. The Nt03 specifically was most potent in all in-vitro assays. The molecular docking studies supported our in-vitro results. It is concluded that N. thomsonianum is a rich source of bioactive antidiabetic compounds which can be further extended to in-vivo based experiments.
Keywords: Antioxidant; Bioactive compounds; Notholirion thomsonianum; Protein tyrosine phosphatase 1B (PTP1B); α-Amylase; α-Glucosidase.
© 2021. The Author(s).
Conflict of interest statement
Dr. Abdul Sadiq and Dr. Muhammad Ayaz are the editorial board members in this journal. All other authors declare that they have no competing interest.
Figures

Similar articles
-
In-vitro, in-vivo and computational experiments for the antidiabetic potential of bioactive compound from Notholirion thomsonianum.Nat Prod Res. 2025 May 28:1-14. doi: 10.1080/14786419.2025.2509158. Online ahead of print. Nat Prod Res. 2025. PMID: 40434871
-
Phytochemical analysis and antidiabetic potential of Elaeagnus umbellata (Thunb.) in streptozotocin-induced diabetic rats: pharmacological and computational approach.BMC Complement Altern Med. 2018 Dec 13;18(1):332. doi: 10.1186/s12906-018-2381-8. BMC Complement Altern Med. 2018. PMID: 30545352 Free PMC article.
-
Phytochemistry, anti-diabetic and antioxidant potentials of Allium consanguineum Kunth.BMC Complement Med Ther. 2022 Jun 13;22(1):154. doi: 10.1186/s12906-022-03639-5. BMC Complement Med Ther. 2022. PMID: 35698061 Free PMC article.
-
Screening of antidiabetic and antioxidant activities of medicinal plants.J Integr Med. 2015 Sep;13(5):297-305. doi: 10.1016/S2095-4964(15)60193-5. J Integr Med. 2015. PMID: 26343100 Review.
-
Plant Products in the Prevention of Diabetes Mellitus.Mini Rev Med Chem. 2022;22(10):1395-1419. doi: 10.2174/1389557521666211116122232. Mini Rev Med Chem. 2022. PMID: 34784862 Review.
Cited by
-
New Succinimide-Thiazolidinedione Hybrids as Multitarget Antidiabetic Agents: Design, Synthesis, Bioevaluation, and Molecular Modelling Studies.Molecules. 2023 Jan 26;28(3):1207. doi: 10.3390/molecules28031207. Molecules. 2023. PMID: 36770873 Free PMC article.
-
3-(((1S,3S)-3-((R)-Hydroxy(4-(trifluoromethyl)phenyl)methyl)-4-oxocyclohexyl)methyl)pentane-2,4-dione: Design and Synthesis of New Stereopure Multi-Target Antidiabetic Agent.Molecules. 2022 May 19;27(10):3265. doi: 10.3390/molecules27103265. Molecules. 2022. PMID: 35630740 Free PMC article.
-
Phytochemical Analysis, α-Glucosidase and Amylase Inhibitory, and Molecular Docking Studies on Persicaria hydropiper L. Leaves Essential Oils.Evid Based Complement Alternat Med. 2022 Jan 19;2022:7924171. doi: 10.1155/2022/7924171. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35096118 Free PMC article.
-
Exploration of Succinimide Derivative as a Multi-Target, Anti-Diabetic Agent: In Vitro and In Vivo Approaches.Molecules. 2023 Feb 7;28(4):1589. doi: 10.3390/molecules28041589. Molecules. 2023. PMID: 36838577 Free PMC article.
-
Chia (Salvia hispanica L.), a Pre-Hispanic Food in the Treatment of Diabetes Mellitus: Hypoglycemic, Antioxidant, Anti-Inflammatory, and Inhibitory Properties of α-Glucosidase and α-Amylase, and in the Prevention of Cardiovascular Disease.Molecules. 2023 Dec 13;28(24):8069. doi: 10.3390/molecules28248069. Molecules. 2023. PMID: 38138560 Free PMC article. Review.
References
-
- Gershell L. Type 2 diabetes market. Nat Rev Drug Discov. 2005;4(5):367–368. - PubMed
-
- Fonseca V. Clinical significance of targeting postprandial and fasting hyperglycemia in managing type 2 diabetes mellitus. Curr Med Res Opinion. 2003;19(7):635–631. - PubMed
-
- Nichols BL, Avery SE, Karnsakul W, Jahoor F, Sen P, Swallow DM, Luginbuehl U, Hahn D, Sterchi EE. Congenital maltase-glucoamylase deficiency associated with lactase and sucrase deficiencies. J Pediatr Gastroenterol Nutr. 2002;35(4):573–579. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

