One year of laboratory-based COVID-19 surveillance system in Belgium: main indicators and performance of the laboratories (March 2020-21)
- PMID: 34706768
- PMCID: PMC8548266
- DOI: 10.1186/s13690-021-00704-2
One year of laboratory-based COVID-19 surveillance system in Belgium: main indicators and performance of the laboratories (March 2020-21)
Abstract
Background: With the spread of coronavirus disease 2019 (COVID-19), an existing national laboratory-based surveillance system was adapted to daily monitor the epidemiological situation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the Belgium by following the number of confirmed SARS-CoV-2 infections, the number of performed tests and the positivity ratio. We present these main indicators of the surveillance over a one-year period as well as the impact of the performance of the laboratories, regarding speed of processing the samples and reporting results, for surveillance.
Methods: We describe the evolution of test capacity, testing strategy and the data collection methods during the first year of the epidemic in Belgium.
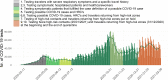
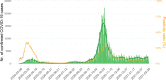
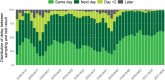
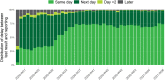
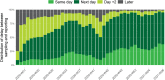
Results: Between the 1st of March 2020 and the 28th of February 2021, 9,487,470 tests and 773,078 COVID-19 laboratory confirmed cases were reported. Two epidemic waves occurred, with a peak in April and October 2020. The capacity and performance of the laboratories improved continuously during 2020 resulting in a high level performance. Since the end of November 2020 90 to 95% of the test results are reported at the latest the day after sampling was performed.
Conclusions: Thanks to the effort of all laboratories a performant exhaustive national laboratory-based surveillance system to monitor the epidemiological situation of SARS-CoV-2 was set up in Belgium in 2020. On top of expanding the number of laboratories performing diagnostics and significantly increasing the test capacity in Belgium, turnaround times between sampling and testing as well as reporting were optimized over the first year of this pandemic.
Keywords: Belgium; COVID-19; Laboratory-based surveillance; SARS-CoV-2.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- World Health Organization . Novel Coronavirus (2019-nCoV) situation report - 1 21 January 2020. 2020.
-
- World Health Organization . Novel Coronavirus (2019-nCoV) situation report - 6 26 January 2020. 2020.
-
- Federal Public Service (FPS) Health, Food Chain Safety and Environment . One repatriated Belgian has tested positive for the novel coronavirus. 2020.
LinkOut - more resources
Full Text Sources
Miscellaneous

